当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of nitric acid concentration for nitration of fused [1,2,5]oxadiazolo[3,4-d]pyrimidine-5,7-diamine
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-11-09 , DOI: 10.1039/d2dt03255d Ajay Kumar Chinnam 1 , Yongxing Tang 2 , Richard J Staples 3 , Jean'ne M Shreeve 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-11-09 , DOI: 10.1039/d2dt03255d Ajay Kumar Chinnam 1 , Yongxing Tang 2 , Richard J Staples 3 , Jean'ne M Shreeve 1
Affiliation
|
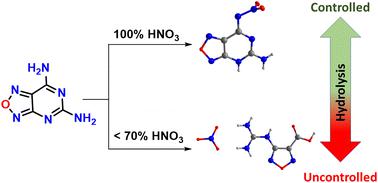
Nitration reactions are very often used for the selective synthesis of novel, high performing nitramine-based materials. Now nitration reactions of the fused 5,7-diamino pyrimidine derivative 1, under different nitric acid concentrations were examined. Concentrated nitric acid gave selectively N-(5-amino-4,5-dihydro-[1,2,5]oxadiazolo[3,4-d]pyrimidin-7-yl)nitramide, 2, while the fused ring nitrate salt, 4, and ring open nitrate salt, 3 were obtained using low concentrations of nitric acid (<70%). In addition, the cesium salt of the fused nitramine derivative 5 was synthesized. All new compounds were isolated in high yields and comprehensively characterized by NMR, FTIR spectroscopy, and elemental analyses. The molecular structures of 2, 3, and 5 were analyzed by single X-ray crystallographic data. These compounds have high calculated heats of formation and high crystal densities. Detonation properties for compounds 2 and 3 were calculated using EXPLO5 software. Fused ring compound 2 (vD, 8549 m s−1; P, 29.62 GPa), and nitrate salt, 3 (vD, 8392 m s−1; P, 29.37 GPa) have superior detonation properties compared with TNT (vD, 7303 m s−1; P, 21.30 GPa). In addition, electrostatic potentials, two-dimensional (2D)-fingerprints, and Hirshfeld surface analysis were used to predict the sensitive properties of compounds 2 and 3. The experimental sensitives suggest possible applications with insensitive energetic applications.
中文翻译:

硝酸浓度对稠合[1,2,5]恶二唑并[3,4-d]嘧啶-5,7-二胺硝化反应的影响
硝化反应经常用于选择性合成新型高性能硝胺基材料。现在检查稠合的5,7-二氨基嘧啶衍生物1在不同硝酸浓度下的硝化反应。浓硝酸选择性地生成N -(5-amino-4,5-dihydro-[1,2,5]oxadiazolo[3,4- d ]pyrimidin-7-yl)nitramide, 2,而稠环硝酸盐,4和开环硝酸盐3是使用低浓度硝酸 (<70%) 获得的。此外,稠合硝胺衍生物5的铯盐被合成了。所有新化合物均以高产率分离,并通过 NMR、FTIR 光谱和元素分析进行了全面表征。通过单次 X 射线晶体学数据分析了2、3和5的分子结构。这些化合物具有高计算生成热和高晶体密度。使用 EXPLO5 软件计算化合物2和3的爆轰特性。稠环化合物2 ( v D , 8549 ms -1 ; P , 29.62 GPa) 和硝酸盐3 ( v D , 8392 ms -1 ;P , 29.37 GPa) 与 TNT ( v D , 7303 ms -1 ; P , 21.30 GPa) 相比具有更好的爆轰特性。此外,静电势、二维 (2D) 指纹和 Hirshfeld 表面分析用于预测化合物2和3的敏感特性。实验敏感性表明可能应用不敏感的高能应用。
更新日期:2022-11-11
中文翻译:

硝酸浓度对稠合[1,2,5]恶二唑并[3,4-d]嘧啶-5,7-二胺硝化反应的影响
硝化反应经常用于选择性合成新型高性能硝胺基材料。现在检查稠合的5,7-二氨基嘧啶衍生物1在不同硝酸浓度下的硝化反应。浓硝酸选择性地生成N -(5-amino-4,5-dihydro-[1,2,5]oxadiazolo[3,4- d ]pyrimidin-7-yl)nitramide, 2,而稠环硝酸盐,4和开环硝酸盐3是使用低浓度硝酸 (<70%) 获得的。此外,稠合硝胺衍生物5的铯盐被合成了。所有新化合物均以高产率分离,并通过 NMR、FTIR 光谱和元素分析进行了全面表征。通过单次 X 射线晶体学数据分析了2、3和5的分子结构。这些化合物具有高计算生成热和高晶体密度。使用 EXPLO5 软件计算化合物2和3的爆轰特性。稠环化合物2 ( v D , 8549 ms -1 ; P , 29.62 GPa) 和硝酸盐3 ( v D , 8392 ms -1 ;P , 29.37 GPa) 与 TNT ( v D , 7303 ms -1 ; P , 21.30 GPa) 相比具有更好的爆轰特性。此外,静电势、二维 (2D) 指纹和 Hirshfeld 表面分析用于预测化合物2和3的敏感特性。实验敏感性表明可能应用不敏感的高能应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号