当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Benzylic C(sp3)–H Bonds Play the Dual Role of Starting Material and Oxidation Inhibitor for Hydrazides in the Electrochemical Synthesis of Hydrazones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-08 , DOI: 10.1021/acs.joc.2c01574 Issa Yavari 1 , Sina Shaabanzadeh 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-08 , DOI: 10.1021/acs.joc.2c01574 Issa Yavari 1 , Sina Shaabanzadeh 1
Affiliation
|
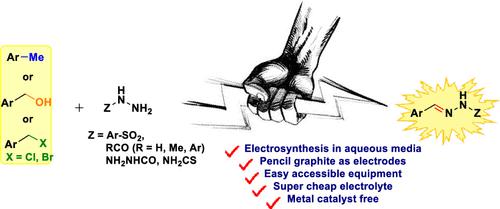
The electrooxidation of benzylic C(sp3)–H bonds to produce hydrazones as an alternate for conventional pathways has an enormous dignity. Under the aegis of electricity, instead of hazardous metal catalysts and external oxidants, we unveil an electrochemical process for electrooxidation of various benzylic C(sp3)–H bonds in aqueous media in all pH ranges that subsequently produce hydrazones with further reactions. This electrooxidative reaction strategy provides an acceptable condition for synthesizing hydrazones with various functional groups in good efficiency and amenable to gram-scale synthesis. The electrochemical oxidation condition proves an excellent level of compatibility with super cheap electrolyte NaCl for the oxidation of benzylic C(sp3)–H position despite the highly oxidizable hydrazide group remaining intact in the reaction.
中文翻译:

苄基 C(sp3)–H 键在酰肼的电化学合成中发挥起始原料和酰肼氧化抑制剂的双重作用
苄基 C(sp 3 )–H 键的电氧化产生腙作为常规途径的替代品具有巨大的尊严。在电力的支持下,而不是有害金属催化剂和外部氧化剂,我们揭示了一种电化学过程,用于在所有 pH 范围内的水介质中电氧化各种苄基 C(sp 3 )–H 键,随后通过进一步反应产生腙。这种电氧化反应策略为高效合成具有各种官能团的腙提供了可接受的条件,并且适合克级合成。电化学氧化条件证明与超级便宜的电解质 NaCl 具有极好的相容性,用于氧化苄基 C(sp 3)–H 位,尽管高度可氧化的酰肼基团在反应中保持完整。
更新日期:2022-11-08
中文翻译:

苄基 C(sp3)–H 键在酰肼的电化学合成中发挥起始原料和酰肼氧化抑制剂的双重作用
苄基 C(sp 3 )–H 键的电氧化产生腙作为常规途径的替代品具有巨大的尊严。在电力的支持下,而不是有害金属催化剂和外部氧化剂,我们揭示了一种电化学过程,用于在所有 pH 范围内的水介质中电氧化各种苄基 C(sp 3 )–H 键,随后通过进一步反应产生腙。这种电氧化反应策略为高效合成具有各种官能团的腙提供了可接受的条件,并且适合克级合成。电化学氧化条件证明与超级便宜的电解质 NaCl 具有极好的相容性,用于氧化苄基 C(sp 3)–H 位,尽管高度可氧化的酰肼基团在反应中保持完整。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号