当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improving Toleration of Volume Expansion of Silicon-Based Anode by Constructing a Flexible Solid-Electrolyte Interface Film via Lithium Difluoro(bisoxalato) Phosphate Electrolyte Additive
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-11-08 , DOI: 10.1021/acssuschemeng.2c04795 Jie Wang 1, 2, 3 , Shiyou Li 1, 2, 3 , Jingjing Zhang 1, 2, 3 , Linhu Song 1, 2, 3 , Hong Dong 1, 2, 3 , Ningshuang Zhang 1, 2, 3 , Peng Wang 1, 2, 3 , Dongni Zhao 1, 2, 3 , Lijuan Zhang 4 , Xiaoling Cui 1, 2, 3
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-11-08 , DOI: 10.1021/acssuschemeng.2c04795 Jie Wang 1, 2, 3 , Shiyou Li 1, 2, 3 , Jingjing Zhang 1, 2, 3 , Linhu Song 1, 2, 3 , Hong Dong 1, 2, 3 , Ningshuang Zhang 1, 2, 3 , Peng Wang 1, 2, 3 , Dongni Zhao 1, 2, 3 , Lijuan Zhang 4 , Xiaoling Cui 1, 2, 3
Affiliation
|
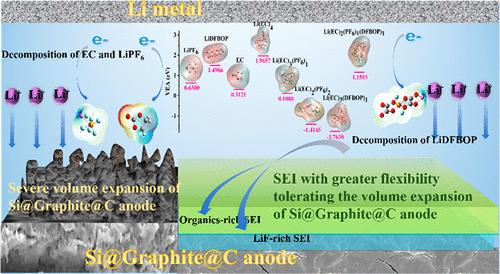
The silicon (Si) anode is considered one of the most promising candidates among many novel anode materials in lithium-ion batteries owing to its high theoretical capacity and earth abundancy. Nonetheless, a large volume expansion of Si particles appears with cycling, prompting unceasing breakage/reformation of the solid-electrolyte interface (SEI) and fast capacity degradation in traditional electrolytes. For the purpose of tolerating volume expansion for the Si anode, lithium difluoro(bisoxalato) phosphate (LiDFBOP) was adopted in the standard (STD) electrolyte based on LiPF6. Density functional theory (DFT) calculations, Young’s modulus from atomic force microscopy, potential-resolved in situ electrochemical impedance spectroscopy (PRI-EIS) measurement, and other characterizations proved that the formed SEI can inhibit volume expansion of a Si@Graphite@C anode. In the STD+2% LiDFBOP electrolyte, the solvation structure of Li(EC)2(PF6)1(DFBOP)1 is more likely to be produced, and this kind of solvation structure has stronger reducibility and easily participates in SEI formation. The 2% LiDFBOP additive increases the inorganic LiF component of SEI, which yields great advantages in regulating the uniform diffusion of Li+ ions passing through the SEI. Besides, the organics containing P and F atoms are also abundant in SEI, which has greater flexibility and can tolerate volume expansion of the Si@Graphite@C anode. Therefore, the STD+2% LiDFBOP electrolyte can improve the electrochemical performances of Si@Graphite@C/Li half-cells. This work has practical implications not only for the molecular design of novel lithium salt but also for the constructions of SEI and electrolyte systems compatible with the Si@Graphite@C anode.
中文翻译:

二氟二草酸磷酸盐电解质添加剂构建柔性固体电解质界面膜提高硅基负极体积膨胀耐受性
硅 (Si) 负极由于其高理论容量和地球丰度,被认为是锂离子电池中众多新型负极材料中最有前途的候选材料之一。尽管如此,随着循环出现 Si 颗粒的大量体积膨胀,促使固体电解质界面 (SEI) 不断破裂/重组,传统电解质的容量快速下降。为了承受 Si 阳极的体积膨胀,在基于 LiPF 6的标准(STD)电解质中采用了二氟(双草酸)磷酸锂(LiDFBOP). 密度泛函理论 (DFT) 计算、原子力显微镜的杨氏模量、电位分辨原位电化学阻抗谱 (PRI-EIS) 测量和其他表征证明,形成的 SEI 可以抑制 Si@Graphite@C 负极的体积膨胀. 在STD+2% LiDFBOP电解液中,更容易产生Li(EC) 2 (PF 6 ) 1 (DFBOP) 1的溶剂化结构,这种溶剂化结构具有更强的还原性,容易参与SEI的形成。2% LiDFBOP 添加剂增加了SEI 的无机LiF 成分,这在调节Li +的均匀扩散方面具有很大优势离子通过 SEI。此外,含有P和F原子的有机物在SEI中也很丰富,具有更大的柔韧性,可以承受Si@Graphite@C负极的体积膨胀。因此,STD+2% LiDFBOP 电解质可以提高 Si@Graphite@C/Li 半电池的电化学性能。这项工作不仅对新型锂盐的分子设计具有实际意义,而且对与 Si@Graphite@C 负极相容的 SEI 和电解质系统的构建也具有实际意义。
更新日期:2022-11-08
中文翻译:

二氟二草酸磷酸盐电解质添加剂构建柔性固体电解质界面膜提高硅基负极体积膨胀耐受性
硅 (Si) 负极由于其高理论容量和地球丰度,被认为是锂离子电池中众多新型负极材料中最有前途的候选材料之一。尽管如此,随着循环出现 Si 颗粒的大量体积膨胀,促使固体电解质界面 (SEI) 不断破裂/重组,传统电解质的容量快速下降。为了承受 Si 阳极的体积膨胀,在基于 LiPF 6的标准(STD)电解质中采用了二氟(双草酸)磷酸锂(LiDFBOP). 密度泛函理论 (DFT) 计算、原子力显微镜的杨氏模量、电位分辨原位电化学阻抗谱 (PRI-EIS) 测量和其他表征证明,形成的 SEI 可以抑制 Si@Graphite@C 负极的体积膨胀. 在STD+2% LiDFBOP电解液中,更容易产生Li(EC) 2 (PF 6 ) 1 (DFBOP) 1的溶剂化结构,这种溶剂化结构具有更强的还原性,容易参与SEI的形成。2% LiDFBOP 添加剂增加了SEI 的无机LiF 成分,这在调节Li +的均匀扩散方面具有很大优势离子通过 SEI。此外,含有P和F原子的有机物在SEI中也很丰富,具有更大的柔韧性,可以承受Si@Graphite@C负极的体积膨胀。因此,STD+2% LiDFBOP 电解质可以提高 Si@Graphite@C/Li 半电池的电化学性能。这项工作不仅对新型锂盐的分子设计具有实际意义,而且对与 Si@Graphite@C 负极相容的 SEI 和电解质系统的构建也具有实际意义。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号