当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Mechanism and Selectivity Tuning of Propene Oxidation at the Electrochemical Interface
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-07 , DOI: 10.1021/jacs.2c09105 Xiao-Chen Liu 1 , Tao Wang 1 , Zhi-Ming Zhang 1 , Cong-Hua Yang 1 , Lai-Yang Li 1 , Shimiao Wu 1 , Shunji Xie 1 , Gang Fu 1 , Zhi-You Zhou 1 , Shi-Gang Sun 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-07 , DOI: 10.1021/jacs.2c09105 Xiao-Chen Liu 1 , Tao Wang 1 , Zhi-Ming Zhang 1 , Cong-Hua Yang 1 , Lai-Yang Li 1 , Shimiao Wu 1 , Shunji Xie 1 , Gang Fu 1 , Zhi-You Zhou 1 , Shi-Gang Sun 1
Affiliation
|
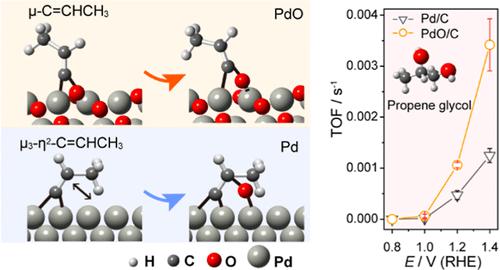
Electrochemical conversion of propene is a promising technique for manufacturing commodity chemicals by using renewable electricity. To achieve this goal, we still need to develop high-performance electrocatalysts for propene electrooxidation, which highly relies on understanding the reaction mechanism at the molecular level. Although the propene oxidation mechanism has been well investigated at the solid/gas interface under thermocatalytic conditions, it still remains elusive at the solid/liquid interface under an electrochemical environment. Here, we report the mechanistic studies of propene electrooxidation on PdO/C and Pd/C catalysts, considering that the Pd-based catalyst is one of the most promising electrocatalytic systems. By electrochemical in situ attenuated total reflection Fourier transform infrared spectroscopy, a distinct reaction pathway was observed compared with conventional thermocatalysis, emphasizing that propene can be dehydrogenated at a potential higher than 0.80 V, and strongly adsorb via μ-C═CHCH3 and μ3-η2-C═CHCH3 configuration on PdO and Pd, respectively. The μ-C═CHCH3 is via bridge bonds on adjacent Pd and O atoms on PdO, and it can be further oxidized by directly taking surface oxygen from PdO, verified by the H218O isotope-edited experiment. A high surface oxygen content on PdO/C results in a 3 times higher turnover frequency than that on Pd/C for converting propene into propene glycol. This finding highlights the different reaction pathways under an electrochemical environment, which sheds light on designing next-generation electrocatalysts for propene electrooxidation.
中文翻译:

丙烯氧化在电化学界面的反应机理和选择性调节
丙烯的电化学转化是利用可再生电力制造商品化学品的有前途的技术。为了实现这一目标,我们仍然需要开发用于丙烯电氧化的高性能电催化剂,这高度依赖于对分子水平反应机理的理解。尽管在热催化条件下在固/气界面上已经很好地研究了丙烯氧化机理,但在电化学环境下在固/液界面上仍然难以捉摸。在这里,我们报告了丙烯电氧化在 PdO/C 和 Pd/C 催化剂上的机理研究,认为 Pd 基催化剂是最有前途的电催化系统之一。通过电化学原位衰减全反射傅里叶变换红外光谱,3和μ 3 -η 2 -C=CHCH 3分别在PdO和Pd上的构型。μ-C=CHCH 3通过PdO上相邻的Pd和O原子的桥键,直接从PdO中夺取表面氧可以进一步氧化,H 2 18 O同位素编辑实验验证了这一点。PdO/C 上的高表面氧含量导致将丙烯转化为丙二醇的转换频率比 Pd/C 上的转换频率高 3 倍。这一发现突出了电化学环境下的不同反应途径,这为设计用于丙烯电氧化的下一代电催化剂提供了启示。
更新日期:2022-11-07
中文翻译:

丙烯氧化在电化学界面的反应机理和选择性调节
丙烯的电化学转化是利用可再生电力制造商品化学品的有前途的技术。为了实现这一目标,我们仍然需要开发用于丙烯电氧化的高性能电催化剂,这高度依赖于对分子水平反应机理的理解。尽管在热催化条件下在固/气界面上已经很好地研究了丙烯氧化机理,但在电化学环境下在固/液界面上仍然难以捉摸。在这里,我们报告了丙烯电氧化在 PdO/C 和 Pd/C 催化剂上的机理研究,认为 Pd 基催化剂是最有前途的电催化系统之一。通过电化学原位衰减全反射傅里叶变换红外光谱,3和μ 3 -η 2 -C=CHCH 3分别在PdO和Pd上的构型。μ-C=CHCH 3通过PdO上相邻的Pd和O原子的桥键,直接从PdO中夺取表面氧可以进一步氧化,H 2 18 O同位素编辑实验验证了这一点。PdO/C 上的高表面氧含量导致将丙烯转化为丙二醇的转换频率比 Pd/C 上的转换频率高 3 倍。这一发现突出了电化学环境下的不同反应途径,这为设计用于丙烯电氧化的下一代电催化剂提供了启示。































 京公网安备 11010802027423号
京公网安备 11010802027423号