Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Easy Access to N-(Pyridin-2-yl)benzamides through Electro-oxidative Ring Opening of 2-Arylimidazo[1,2-a]pyridines
Synlett ( IF 1.7 ) Pub Date : 2022-11-07 , DOI: 10.1055/a-1956-9993 Sayan Ghosh 1 , Jhilik Dutta 1 , Atreyee Halder 1 , Suman De Sarkar 1
中文翻译:

通过 2-芳基咪唑并[1,2-a]吡啶的电氧化开环容易获得 N-(Pyridin-2-yl)benzamides
更新日期:2022-11-08
Synlett ( IF 1.7 ) Pub Date : 2022-11-07 , DOI: 10.1055/a-1956-9993 Sayan Ghosh 1 , Jhilik Dutta 1 , Atreyee Halder 1 , Suman De Sarkar 1
Affiliation
|
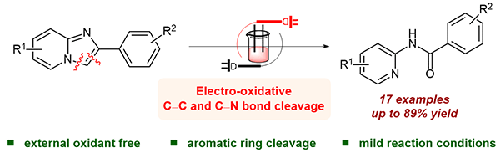
An electro-oxidative method for the ring opening of imidazopyridine derivatives is reported. This mild protocol offers a sustainable alternative to the existing harsh reaction conditions and unleashes an efficient approach to produce N-(pyridin-2-yl)amide derivatives with good tolerance of different functional groups. Systematic mechanistic studies provided insight into the reaction pathway and revealed that the residual water of DMSO is the source of oxygen atoms in the products.
中文翻译:

通过 2-芳基咪唑并[1,2-a]吡啶的电氧化开环容易获得 N-(Pyridin-2-yl)benzamides
报道了一种用于咪唑并吡啶衍生物开环的电氧化方法。这种温和的协议为现有的苛刻反应条件提供了一种可持续的替代方案,并释放了一种有效的方法来生产对不同官能团具有良好耐受性的N- (pyridin-2-yl)amide 衍生物。系统的机理研究提供了对反应途径的深入了解,并揭示了 DMSO 的残留水是产物中氧原子的来源。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号