当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Practical and Facile Access to Bicyclo[3.1.1]heptanes: Potent Bioisosteres of meta-Substituted Benzenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-07 , DOI: 10.1021/jacs.2c09733 Toranosuke Iida 1 , Junichiro Kanazawa 1 , Tadafumi Matsunaga 1 , Kazunori Miyamoto 1 , Keiichi Hirano 1 , Masanobu Uchiyama 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-07 , DOI: 10.1021/jacs.2c09733 Toranosuke Iida 1 , Junichiro Kanazawa 1 , Tadafumi Matsunaga 1 , Kazunori Miyamoto 1 , Keiichi Hirano 1 , Masanobu Uchiyama 1, 2
Affiliation
|
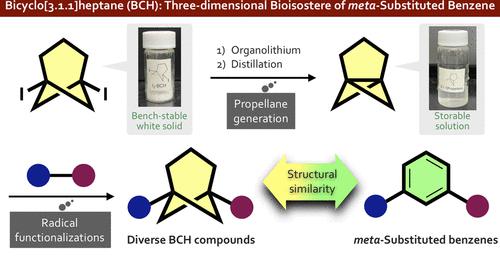
There is increasing interest in replacement of the planar aromatic rings of drug candidates with three-dimensional caged scaffolds in order to improve the physical properties, but bioisosteres of meta-substituted benzenes have remained elusive. We focused on the bicyclo[3.1.1]heptane (BCH) scaffold as a novel bioisostere of meta-substituted benzenes, anticipating that [3.1.1]propellane (2) would be a versatile precursor of diversely functionalized BCHs. Here, we describe a practical preparative method for [3.1.1]propellane from newly developed 1,5-diiodobicyclo[3.1.1]heptane (1), as well as difunctionalization reactions of 2 leading to functionalized BCHs. We also report postfunctionalization reactions of these products.
中文翻译:

双环[3.1.1]庚烷的实用和简便获取:间位取代苯的有效生物电子等排体
人们越来越关注用三维笼状支架取代候选药物的平面芳环以改善物理性质,但间位取代苯的生物电子等排体仍然难以捉摸。我们将双环[3.1.1]庚烷 (BCH) 支架作为间位取代苯的新型生物电子等排体,预计 [3.1.1] 丙烷 ( 2 ) 将成为多种功能化 BCH 的多功能前体。在这里,我们描述了从新开发的 1,5-二碘双环 [3.1.1] 庚烷 ( 1 ) 制备 [3.1.1] 丙烷的实用方法,以及2的双官能化反应导致功能化的 BCH。我们还报告了这些产品的后功能化反应。
更新日期:2022-11-07
中文翻译:

双环[3.1.1]庚烷的实用和简便获取:间位取代苯的有效生物电子等排体
人们越来越关注用三维笼状支架取代候选药物的平面芳环以改善物理性质,但间位取代苯的生物电子等排体仍然难以捉摸。我们将双环[3.1.1]庚烷 (BCH) 支架作为间位取代苯的新型生物电子等排体,预计 [3.1.1] 丙烷 ( 2 ) 将成为多种功能化 BCH 的多功能前体。在这里,我们描述了从新开发的 1,5-二碘双环 [3.1.1] 庚烷 ( 1 ) 制备 [3.1.1] 丙烷的实用方法,以及2的双官能化反应导致功能化的 BCH。我们还报告了这些产品的后功能化反应。






























 京公网安备 11010802027423号
京公网安备 11010802027423号