Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2022-11-07 , DOI: 10.1016/j.bmcl.2022.129049
Shuhei Ogawa 1 , Yasunobu Asawa 2 , Momoka Iiyama 3 , Atsushi Yoshimori 4 , Hiroyuki Nakamura 2 , Masayuki Oda 3
|
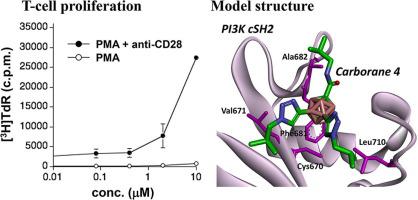
Binding of adaptor molecules, such as growth factor receptor-bound protein 2 (Grb2) and phosphoinositide 3-kinase (PI3K), to the cytoplasmic region of CD28 is critical for T-cell activation. The Src homology 2 (SH2) domains of Grb2 and PI3K interact with the cytoplasmic region, including phosphorylated Tyr, of CD28. We found that trisubstituted carboranes efficiently increased the proliferation of T cells obtained from C57BL/6 mice. The carboranes specifically increased the binding of Grb2 Src homology 2 (SH2) to CD28-derived phosphopeptide but decreased the binding of PI3K C-terminal SH2 (cSH2). Based on the crystal structures of CD28-derived phosphopeptides complexed with Grb2 SH2 and PI3K cSH2, the bound structures of compound 4 (CRL266481) were modeled to determine the molecular mechanism of the regulation.
中文翻译:

三取代碳硼烷调节 CD28 与 Grb2 和 PI3K 的 SH2 结构域结合以激活 T 细胞
诸如生长因子受体结合蛋白 2 (Grb2) 和磷酸肌醇 3 激酶 (PI3K) 等衔接分子与 CD28 细胞质区域的结合对于T细胞活化至关重要。Grb2 和 PI3K 的 Src 同源 2 (SH2) 结构域与细胞质区域相互作用,包括 CD28 的磷酸化 Tyr。我们发现三取代的碳硼烷有效地增加了从 C57BL/6 小鼠获得的 T 细胞的增殖。碳硼烷特别增加了 Grb2 Src 同源性 2 (SH2) 与 CD28 衍生的磷酸肽的结合,但降低了 PI3K C 末端 SH2 (cSH2) 的结合。基于与 Grb2 SH2 和 PI3K cSH2 复合的 CD28 衍生磷酸肽的晶体结构,化合物4的结合结构(CRL266481) 被建模以确定调节的分子机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号