Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2022-11-03 , DOI: 10.1016/j.molstruc.2022.134479 Mahmoud Bazrafshan , Mohammad Vakili , Sayyed Faramarz Tayyari , Fadhil S. Kamounah , Poul Erik Hansen , Ali Shiri
|
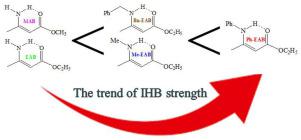
Amino derivatives of ethyl acetoacetate like ethyl (Z)- 3-(amino)but-2-enoate (EAB), ethyl (Z)- 3-(methylamino)but-2-enoate (Me-EAB), ethyl (Z)- 3-(phenylamino)but-2-enoate (Ph-EAB), and ethyl (Z)- 3-(benzylamino)but-2-enoate (Bn-EAB) were synthesized. Their structures, conformations, and intramolecular hydrogen bonding (IHB) is characterized using computational analysis (density functional theory, DFT, methods) at the B3LYP/6-311++G(d,p) level and spectroscopic techniques)IR, UV, and NMR). All the mentioned theoretical and experimental results were compared to those of (Z)-methyl 3-aminobut-2-enoate (MAB). The vibrational spectra of EAB are fully assigned. Vibrational spectroscopy results confirmed the existence of two conformers of title molecules. DFT calculations at the B3LYP/6-311++G(d,p) level show the calculated N H bond lengths in EAB, Me-EAB, Ph-EAB, Bn-EAB, and MAB are 1.015, 1.017, 1.022, 1.018, and 1.014 Ǻ, respectively. Also, according to the natural bond orbital (NBO) results, the σN-H10↔LP(2)O7 interactions for EAB, Me-EAB, Ph-EAB, Bn-EAB, and MAB are 5.21, 6.77, 8.65, 7.18, and 5.11 kcal/mol, respectively. So, the trend in IHB strength is: Ph-EAB > Bn-EAB ∼ Me-EAB > EAB ∼ MAB, which also agrees with the mentioned experimental results. The mentioned trend also is confirmed by the LP(O7)↔σN
H bond lengths in EAB, Me-EAB, Ph-EAB, Bn-EAB, and MAB are 1.015, 1.017, 1.022, 1.018, and 1.014 Ǻ, respectively. Also, according to the natural bond orbital (NBO) results, the σN-H10↔LP(2)O7 interactions for EAB, Me-EAB, Ph-EAB, Bn-EAB, and MAB are 5.21, 6.77, 8.65, 7.18, and 5.11 kcal/mol, respectively. So, the trend in IHB strength is: Ph-EAB > Bn-EAB ∼ Me-EAB > EAB ∼ MAB, which also agrees with the mentioned experimental results. The mentioned trend also is confirmed by the LP(O7)↔σN H steric exchange interaction.
H steric exchange interaction.
中文翻译:

3-氨基-2-丁烯酸乙酯及其N-Me、N-Ph和N-Bn类似物的合成、分子结构、构象和分子内氢键强度;实验和理论研究
乙酰乙酸乙酯的氨基衍生物,如 (Z)-3-(氨基)丁-2-烯酸乙酯 (EAB)、(Z)-3-(甲氨基)丁-2-烯酸乙酯 (Me-EAB)、(Z) - 3-(苯基氨基)丁-2-烯酸酯(Ph-EAB)和乙基(Z)-3-(苄基氨基)丁-2-烯酸酯(Bn-EAB)被合成。它们的结构、构象和分子内氢键 (IHB) 使用 B3LYP/6-311++G(d,p) 水平的计算分析(密度泛函理论、DFT、方法)和光谱技术表征)IR、UV、和核磁共振)。所有提到的理论和实验结果都与 (Z)-甲基 3-氨基丁-2-烯酸酯 (MAB) 的结果进行了比较。EAB 的振动光谱是完全指定的。振动光谱结果证实存在两个标题分子的构象异构体。B3LYP/6-311++G(d,p) 级别的 DFT 计算显示计算的 N EAB、Me-EAB、Ph-EAB、Bn-EAB 和 MAB 中的 H 键长分别为 1.015、1.017、1.022、1.018 和 1.014 ε。此外,根据自然键轨道 (NBO) 结果,EAB、Me-EAB、Ph-EAB、Bn-EAB 和 MAB 的 σN-H10↔LP(2)O7 相互作用分别为 5.21、6.77、8.65、7.18、和 5.11 kcal/mol,分别。因此,IHB 强度的趋势是:Ph-EAB > Bn-EAB ∼ Me-EAB > EAB ∼ MAB,这也与上述实验结果一致。
EAB、Me-EAB、Ph-EAB、Bn-EAB 和 MAB 中的 H 键长分别为 1.015、1.017、1.022、1.018 和 1.014 ε。此外,根据自然键轨道 (NBO) 结果,EAB、Me-EAB、Ph-EAB、Bn-EAB 和 MAB 的 σN-H10↔LP(2)O7 相互作用分别为 5.21、6.77、8.65、7.18、和 5.11 kcal/mol,分别。因此,IHB 强度的趋势是:Ph-EAB > Bn-EAB ∼ Me-EAB > EAB ∼ MAB,这也与上述实验结果一致。 LP(O7)↔σN H 空间交换相互作用也证实了上述趋势。
LP(O7)↔σN H 空间交换相互作用也证实了上述趋势。






























 京公网安备 11010802027423号
京公网安备 11010802027423号