当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Switching on the Electrocatalytic Ethene Epoxidation on Nanocrystalline RuO2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-04-20 , DOI: 10.1021/ja109955w
Jakub S. Jirkovský 1, 2 , Michael Busch 2 , Elisabet Ahlberg 2 , Itai Panas 3 , Petr Krtil 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-04-20 , DOI: 10.1021/ja109955w
Jakub S. Jirkovský 1, 2 , Michael Busch 2 , Elisabet Ahlberg 2 , Itai Panas 3 , Petr Krtil 1
Affiliation
|
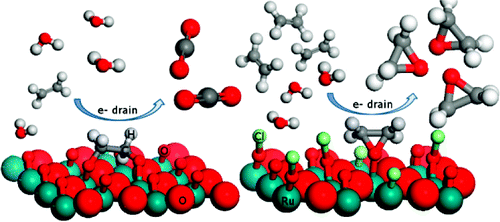
Ruthenium-based oxides with rutile structure were examined regarding their properties in electrocatalytic ethene oxidation in acid media. A possible promoting effect of chloride ions toward oxirane formation was explored. Online differential electrochemical mass spectrometry combined with electrochemical polarization techniques were used to monitor the potential dependence of organic products resulting from ethene oxidation as well as the reaction solution decomposition products. Quantum chemical modeling by means of density functional theory was employed to study key reaction steps. The ethene oxidation in acid media led to CO(2), whereas oxirane was formed in the presence of 0.3 M Cl(-). In the Cl(-) promoted oxidation on RuO(2), oxirane and a small amount of CO(2) were the only detected electro-oxidation products at potentials below the onset of Cl(2) and O(2) evolution, resulting from Cl(-) and water oxidation. It is demonstrated here that the epoxidation is a surface-related electrocatalytic process that depends on the surface properties. Cl acts as the epoxidation promoter that switches off the combustion pathway toward CO(2) and enables the epoxidation reaction channel by surface reactive sites blocking. The proposed epoxidation mechanism implies binuclear (recombination) mechanism for O(2) evolution reaction on considered surfaces.
中文翻译:

在纳米晶 RuO2 上开启电催化乙烯环氧化反应
研究了具有金红石结构的钌基氧化物在酸性介质中电催化乙烯氧化的性能。探索了氯离子对环氧乙烷形成的可能促进作用。在线差分电化学质谱与电化学极化技术相结合,用于监测乙烯氧化产生的有机产物以及反应溶液分解产物的电位依赖性。采用密度泛函理论的量子化学建模来研究关键反应步骤。酸性介质中的乙烯氧化导致 CO(2),而环氧乙烷是在 0.3 M Cl(-) 存在的情况下形成的。在 Cl(-) 促进了 RuO(2) 上的氧化,环氧乙烷和少量的 CO(2) 是唯一检测到的电氧化产物,其电位低于 Cl(2) 和 O(2) 演化的开始,由 Cl(-) 和水氧化引起。这里证明环氧化是一种与表面相关的电催化过程,取决于表面性质。Cl 充当环氧化促进剂,关闭燃烧路径朝向 CO(2) 并通过表面反应位点阻塞启用环氧化反应通道。建议的环氧化机制意味着 O(2) 演化反应在考虑的表面上的双核(重组)机制。Cl 充当环氧化促进剂,关闭通向 CO(2) 的燃烧路径,并通过表面活性位点阻塞启用环氧化反应通道。建议的环氧化机制意味着 O(2) 演化反应在考虑的表面上的双核(重组)机制。Cl 充当环氧化促进剂,关闭燃烧路径朝向 CO(2) 并通过表面反应位点阻塞启用环氧化反应通道。建议的环氧化机制意味着 O(2) 演化反应在考虑的表面上的双核(重组)机制。
更新日期:2011-04-20
中文翻译:

在纳米晶 RuO2 上开启电催化乙烯环氧化反应
研究了具有金红石结构的钌基氧化物在酸性介质中电催化乙烯氧化的性能。探索了氯离子对环氧乙烷形成的可能促进作用。在线差分电化学质谱与电化学极化技术相结合,用于监测乙烯氧化产生的有机产物以及反应溶液分解产物的电位依赖性。采用密度泛函理论的量子化学建模来研究关键反应步骤。酸性介质中的乙烯氧化导致 CO(2),而环氧乙烷是在 0.3 M Cl(-) 存在的情况下形成的。在 Cl(-) 促进了 RuO(2) 上的氧化,环氧乙烷和少量的 CO(2) 是唯一检测到的电氧化产物,其电位低于 Cl(2) 和 O(2) 演化的开始,由 Cl(-) 和水氧化引起。这里证明环氧化是一种与表面相关的电催化过程,取决于表面性质。Cl 充当环氧化促进剂,关闭燃烧路径朝向 CO(2) 并通过表面反应位点阻塞启用环氧化反应通道。建议的环氧化机制意味着 O(2) 演化反应在考虑的表面上的双核(重组)机制。Cl 充当环氧化促进剂,关闭通向 CO(2) 的燃烧路径,并通过表面活性位点阻塞启用环氧化反应通道。建议的环氧化机制意味着 O(2) 演化反应在考虑的表面上的双核(重组)机制。Cl 充当环氧化促进剂,关闭燃烧路径朝向 CO(2) 并通过表面反应位点阻塞启用环氧化反应通道。建议的环氧化机制意味着 O(2) 演化反应在考虑的表面上的双核(重组)机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号