当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Role of Hydroxyl Groups on Cu/Al2O3 in CO2 Hydrogenation
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-11-04 , DOI: 10.1021/acscatal.2c03591
Xiwen Song 1 , Chengsheng Yang 1 , Xianghong Li 1 , Zhongyan Wang 1 , Chunlei Pei 1 , Zhi-Jian Zhao 1 , Jinlong Gong 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-11-04 , DOI: 10.1021/acscatal.2c03591
Xiwen Song 1 , Chengsheng Yang 1 , Xianghong Li 1 , Zhongyan Wang 1 , Chunlei Pei 1 , Zhi-Jian Zhao 1 , Jinlong Gong 1, 2
Affiliation
|
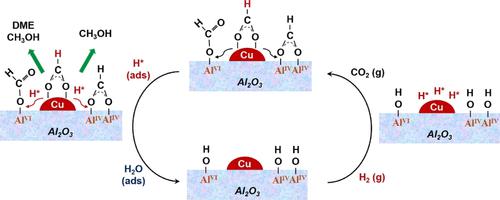
Understanding the nature of adsorbates and intermediates on catalytic surfaces is critical for the development of highly efficient systems for CO2 conversion. However, the role of structural hydroxyl groups in CO2 hydrogenation is still under debate. This paper describes an experimental study on the nature of surface-bound hydroxyl groups over Al2O3-supported Cu catalysts for CO2 reduction. A model 5 wt % Cu/Al2O3 catalyst with stable hydroxyl groups was prepared. The structural hydroxyl groups were modulated by the hydration of unsaturated Al3+ sites, which were introduced by the evaporation-induced self-assembly method. Terminal hydroxyl groups (T-OH*) were found to significantly facilitate the conversion of CO2, while the AlVI-T-OH and AlIV-T-OH species promoted the formation of Al-b-HCOO and Al-m-HCOO species, respectively, accompanied with the increase in methanol and dimethyl ether yields. The reaction behavior of hydroxyl species was further investigated by in situ IR experiments with HD isotope exchange on Cu/Al2O3. T-OH* were identified as active sites participating in the formation of key intermediates, while more hydroxyl sites were occupied by formate species with the H coadsorbed on the Cu surfaces.
中文翻译:

Cu/Al2O3 上羟基在 CO2 加氢中的作用
了解催化表面上吸附物和中间体的性质对于开发高效的 CO 2转化系统至关重要。然而,结构羟基在CO 2加氢中的作用仍在争论中。该论文描述了用于 CO 2还原的 Al 2 O 3负载的铜催化剂表面结合羟基的性质的实验研究。制备了具有稳定羟基的模型 5 wt% Cu/Al 2 O 3催化剂。结构羟基由不饱和Al 3+的水合调节通过蒸发诱导自组装方法引入的位点。发现末端羟基 (T-OH*) 显着促进了 CO 2的转化,而 Al VI -T-OH 和 Al IV -T-OH 物质促进了 Al- b -HCOO 和 Al- m -的形成HCOO 物种分别伴随着甲醇和二甲醚产率的增加。通过在 Cu/Al 2 O 3上进行 HD 同位素交换的原位红外实验,进一步研究了羟基物质的反应行为. T-OH* 被确定为参与关键中间体形成的活性位点,而更多的羟基位点被甲酸盐物种占据,H 共吸附在 Cu 表面上。
更新日期:2022-11-04
中文翻译:

Cu/Al2O3 上羟基在 CO2 加氢中的作用
了解催化表面上吸附物和中间体的性质对于开发高效的 CO 2转化系统至关重要。然而,结构羟基在CO 2加氢中的作用仍在争论中。该论文描述了用于 CO 2还原的 Al 2 O 3负载的铜催化剂表面结合羟基的性质的实验研究。制备了具有稳定羟基的模型 5 wt% Cu/Al 2 O 3催化剂。结构羟基由不饱和Al 3+的水合调节通过蒸发诱导自组装方法引入的位点。发现末端羟基 (T-OH*) 显着促进了 CO 2的转化,而 Al VI -T-OH 和 Al IV -T-OH 物质促进了 Al- b -HCOO 和 Al- m -的形成HCOO 物种分别伴随着甲醇和二甲醚产率的增加。通过在 Cu/Al 2 O 3上进行 HD 同位素交换的原位红外实验,进一步研究了羟基物质的反应行为. T-OH* 被确定为参与关键中间体形成的活性位点,而更多的羟基位点被甲酸盐物种占据,H 共吸附在 Cu 表面上。































 京公网安备 11010802027423号
京公网安备 11010802027423号