当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
FeS2@TiO2 nanobelt array enabled high-efficiency electrocatalytic nitrate reduction to ammonia
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-11-04 , DOI: 10.1039/d2ta07475c
Haipeng Wang 1, 2 , Donglin Zhao 2 , Chaozhen Liu 3 , Xiaoya Fan 2 , Zerong Li 2 , Yongsong Luo 2 , Dongdong Zheng 2 , Shengjun Sun 4 , Jie Chen 5 , Jing Zhang 6 , Yang Liu 7 , Shuyan Gao 7 , Feng Gong 3 , Xuping Sun 2, 4
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-11-04 , DOI: 10.1039/d2ta07475c
Haipeng Wang 1, 2 , Donglin Zhao 2 , Chaozhen Liu 3 , Xiaoya Fan 2 , Zerong Li 2 , Yongsong Luo 2 , Dongdong Zheng 2 , Shengjun Sun 4 , Jie Chen 5 , Jing Zhang 6 , Yang Liu 7 , Shuyan Gao 7 , Feng Gong 3 , Xuping Sun 2, 4
Affiliation
|
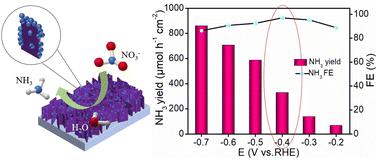
Nitrate (NO3−), which exists in both surface water and underground water, is harmful to the environment and human body. Ammonia (NH3) is a kind of necessary produced chemical for industry and daily life. Electrochemical NO3− reduction can eliminate hazardous NO3− and produce value-added NH3 at the same time under ambient conditions but requires efficient catalysts for the NO3− reduction reaction (NO3RR) with high selectivity. Herein, we report on the development of FeS2 nanoparticles on TiO2 nanobelt array (FeS2@TiO2) as an earth-abundant NO3RR electrocatalyst. It shows superior electrocatalytic performance with a large NH3 yield of 860.3 μmol h−1 cm−2 at −0.7 V and a high faradaic efficiency of 97.0% at −0.4 V versus reversible hydrogen electrode in 0.1 M NaOH with 0.1 M NO3−. The theoretical calculations reveal the mechanisms of the enhanced NO3RR performance of FeS2@TiO2.
中文翻译:

FeS2@TiO2 纳米带阵列实现高效电催化硝酸盐还原为氨
硝酸盐(NO 3 - )存在于地表水和地下水中,对环境和人体有害。氨(NH 3 )是工业和日常生活必需生产的化学品。电化学 NO 3 -还原可以在环境条件下同时消除有害的 NO 3 -并产生增值的 NH 3,但需要高效的催化剂来进行高选择性的 NO 3 -还原反应 (NO 3 RR)。在此,我们报告了在 TiO 2纳米带阵列 ( FeS 2 @TiO 2) 作为地球上丰富的 NO 3 RR 电催化剂。它显示出优异的电催化性能,在 0.1 M NaOH 和 0.1 M NO 3 −中,NH 3产率高达 860.3 μmol h −1 cm −2,在 −0.4 V 时法拉第效率高达 97.0%,与可逆氢电极相比。 . 理论计算揭示了FeS 2 @TiO 2增强NO 3 RR性能的机制。
更新日期:2022-11-04
中文翻译:

FeS2@TiO2 纳米带阵列实现高效电催化硝酸盐还原为氨
硝酸盐(NO 3 - )存在于地表水和地下水中,对环境和人体有害。氨(NH 3 )是工业和日常生活必需生产的化学品。电化学 NO 3 -还原可以在环境条件下同时消除有害的 NO 3 -并产生增值的 NH 3,但需要高效的催化剂来进行高选择性的 NO 3 -还原反应 (NO 3 RR)。在此,我们报告了在 TiO 2纳米带阵列 ( FeS 2 @TiO 2) 作为地球上丰富的 NO 3 RR 电催化剂。它显示出优异的电催化性能,在 0.1 M NaOH 和 0.1 M NO 3 −中,NH 3产率高达 860.3 μmol h −1 cm −2,在 −0.4 V 时法拉第效率高达 97.0%,与可逆氢电极相比。 . 理论计算揭示了FeS 2 @TiO 2增强NO 3 RR性能的机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号