当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio- and Enantioselective Synthesis of Dihydropyrido[1,2-a]indoles via Catalytic Asymmetric Annulative Allylic Alkylation
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-02 , DOI: 10.1021/acs.joc.2c01873 Xue Wang 1 , Hui-Lin Mao 1 , Yu-Heng Yang 1 , Hong Jiang 2 , Ling-Qi Chen 1 , Shu-Jiang Tu 1 , Wen-Juan Hao 1 , Bo Jiang 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-02 , DOI: 10.1021/acs.joc.2c01873 Xue Wang 1 , Hui-Lin Mao 1 , Yu-Heng Yang 1 , Hong Jiang 2 , Ling-Qi Chen 1 , Shu-Jiang Tu 1 , Wen-Juan Hao 1 , Bo Jiang 1
Affiliation
|
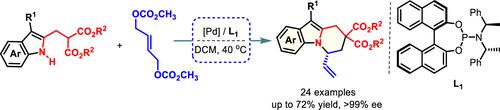
A palladium-catalyzed asymmetric annulative allylic alkylation reaction of 2-[(1H-indol-2-yl)methyl]malonates with (E)-but-2-ene-1,4-diyl dicarbonates is described, leading to the regio- and enantioselective synthesis of dihydropyrido[1,2-a]indoles with a chiral cyclic allyl stereocenter adjacent to the ring-junction nitrogen atom in moderate to good yields. The salient features of this protocol include mild conditions, a broad substrate scope, and good compatibility with substituents as well as high regio- and stereoselectivities, providing a catalytic asymmetric entry for fabricating chiral pyridoindole scaffolds.
中文翻译:

催化不对称环烯丙基烷基化区域选择性和对映体选择性合成二氢吡啶并[1,2-a]吲哚
描述了 2-[(1 H -indol-2-yl)methyl] 丙二酸酯与 ( E )-but-2-ene-1,4-diyl dicarbonates的钯催化不对称环烯丙基烷基化反应,导致区域- 和对映选择性合成二氢吡啶并 [1,2- a ] 吲哚,其具有与环连接氮原子相邻的手性环状烯丙基立体中心,收率中等至良好。该协议的显着特点包括温和的条件、广泛的底物范围、与取代基的良好相容性以及高区域选择性和立体选择性,为制造手性吡啶并吲哚支架提供催化不对称入口。
更新日期:2022-11-02
中文翻译:

催化不对称环烯丙基烷基化区域选择性和对映体选择性合成二氢吡啶并[1,2-a]吲哚
描述了 2-[(1 H -indol-2-yl)methyl] 丙二酸酯与 ( E )-but-2-ene-1,4-diyl dicarbonates的钯催化不对称环烯丙基烷基化反应,导致区域- 和对映选择性合成二氢吡啶并 [1,2- a ] 吲哚,其具有与环连接氮原子相邻的手性环状烯丙基立体中心,收率中等至良好。该协议的显着特点包括温和的条件、广泛的底物范围、与取代基的良好相容性以及高区域选择性和立体选择性,为制造手性吡啶并吲哚支架提供催化不对称入口。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号