当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of N-Methoxy-(biphenyl-ethyl)-pyrazole-carboxamides as Novel Succinate Dehydrogenase Inhibitors
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-11-01 , DOI: 10.1021/acs.jafc.2c04770 Yuan-Hui Huang 1 , Ge Wei 1 , Zheng Liu 1 , Qiang Lu 1 , Jia-Jia Jiang 1 , Xiao-Lei Zhu 1 , Guang-Fu Yang 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-11-01 , DOI: 10.1021/acs.jafc.2c04770 Yuan-Hui Huang 1 , Ge Wei 1 , Zheng Liu 1 , Qiang Lu 1 , Jia-Jia Jiang 1 , Xiao-Lei Zhu 1 , Guang-Fu Yang 1, 2
Affiliation
|
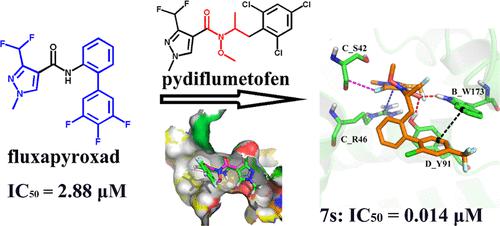
Succinate dehydrogenase (SDH) inhibitor is one of the research hotspots for the development of fungicides. Herein, we describe the design and synthesis of N-methoxy-(biphenyl-ethyl)-pyrazole-carboxamide derivatives with enhanced fungicidal activity by employing fragment combination strategy. The SDH enzymatic activity was evaluated for 24 title compounds, and compound 7s was identified as the highest activity against porcine SDH with an IC50 value of 0.014 μM, 205-fold greater than that of fluxapyroxad. Furthermore, the greenhouse experiments showed that compound 7u exhibited potent fungicidal activity against wheat powdery mildew with an EC50 value of 0.633 mg/L, higher activity than fluxapyroxad and benzovindiflupyr. The computational results showed that the fluorine atom substituted on the pyrazole ring formed an extra dipolar–dipolar interaction with C_S42 and then increased the van der Waals interaction between the compound and SDH. The structural and mechanistic insights obtained from the present work will provide a valuable clue to developing novel SDH inhibitors.
中文翻译:

N-甲氧基-(联苯-乙基)-吡唑-甲酰胺作为新型琥珀酸脱氢酶抑制剂的发现
琥珀酸脱氢酶(SDH)抑制剂是杀菌剂开发的研究热点之一。在此,我们描述了通过采用片段组合策略设计和合成具有增强杀菌活性的N-甲氧基-(联苯-乙基)-吡唑-甲酰胺衍生物。对 24 种标题化合物的 SDH 酶活性进行了评估,化合物7s被鉴定为对猪 SDH 的最高活性,其 IC 50值为 0.014 μM,比 fluxapyroxad 高 205 倍。此外,温室试验表明,化合物7u对小麦白粉病具有有效的杀真菌活性,EC为 50值为 0.633 mg/L,活性高于 fluxapyroxad 和 benzovindiflupyr。计算结果表明,取代在吡唑环上的氟原子与C_S42形成了额外的偶极-偶极相互作用,进而增加了化合物与SDH之间的范德华相互作用。从目前的工作中获得的结构和机制见解将为开发新型 SDH 抑制剂提供有价值的线索。
更新日期:2022-11-01
中文翻译:

N-甲氧基-(联苯-乙基)-吡唑-甲酰胺作为新型琥珀酸脱氢酶抑制剂的发现
琥珀酸脱氢酶(SDH)抑制剂是杀菌剂开发的研究热点之一。在此,我们描述了通过采用片段组合策略设计和合成具有增强杀菌活性的N-甲氧基-(联苯-乙基)-吡唑-甲酰胺衍生物。对 24 种标题化合物的 SDH 酶活性进行了评估,化合物7s被鉴定为对猪 SDH 的最高活性,其 IC 50值为 0.014 μM,比 fluxapyroxad 高 205 倍。此外,温室试验表明,化合物7u对小麦白粉病具有有效的杀真菌活性,EC为 50值为 0.633 mg/L,活性高于 fluxapyroxad 和 benzovindiflupyr。计算结果表明,取代在吡唑环上的氟原子与C_S42形成了额外的偶极-偶极相互作用,进而增加了化合物与SDH之间的范德华相互作用。从目前的工作中获得的结构和机制见解将为开发新型 SDH 抑制剂提供有价值的线索。

































 京公网安备 11010802027423号
京公网安备 11010802027423号