当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multicomponent Cascade Reaction of 3-Cyanochromones: Highly Site-Selective Synthesis of 2-(1H-Imidazol-1-yl)-4H-chromen-4-one Derivatives
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-01 , DOI: 10.1021/acs.joc.2c01719 Ying Lv 1 , Li Chen 1 , Kun Li 1 , Xing-Han Yun 1 , Sheng-Jiao Yan 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-01 , DOI: 10.1021/acs.joc.2c01719 Ying Lv 1 , Li Chen 1 , Kun Li 1 , Xing-Han Yun 1 , Sheng-Jiao Yan 1
Affiliation
|
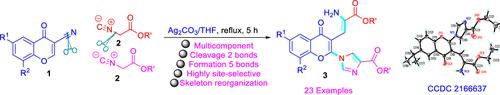
A method for the synthesis of 2-(1H-imidazol-1-yl)-4H-chromen-4-one derivatives (IMCMs) from 3-cyanochromones and α-isocyanoacetates via a one-pot cascade reaction involving a 1,2-addition, Michael reaction, ring-closing, tautomerization, ring-opening, and [3 + 2] cyclization was enabled by refluxing a mixture of the starting materials in THF in the presence of Ag2CO3 as a catalyst. The cascade reaction resulted in the formation of five bonds and the cleavage of two bonds, including a triple bond, in one pot. This protocol enabled not only the synthesis of functionalized imidazoles (i.e., IMCMs) but also the synthesis of functionally useful enamine building blocks. This strategy is suitable for combinatorial and parallel syntheses of IMCMs.
中文翻译:

3-氰色酮的多组分级联反应:2-(1H-咪唑-1-基)-4H-chromen-4-one 衍生物的高位点选择性合成
一种通过涉及1 , 2-加成、Michael 反应、闭环、互变异构、开环和 [3 + 2] 环化通过在 Ag 2 CO 3存在下回流原料在 THF 中的混合物来实现作为催化剂。级联反应导致五个键的形成和两个键的裂解,包括一个三键,在一个锅中。该协议不仅可以合成功能化咪唑(即 IMCM),还可以合成功能有用的烯胺构件。该策略适用于 IMCM 的组合和并行合成。
更新日期:2022-11-01
中文翻译:

3-氰色酮的多组分级联反应:2-(1H-咪唑-1-基)-4H-chromen-4-one 衍生物的高位点选择性合成
一种通过涉及1 , 2-加成、Michael 反应、闭环、互变异构、开环和 [3 + 2] 环化通过在 Ag 2 CO 3存在下回流原料在 THF 中的混合物来实现作为催化剂。级联反应导致五个键的形成和两个键的裂解,包括一个三键,在一个锅中。该协议不仅可以合成功能化咪唑(即 IMCM),还可以合成功能有用的烯胺构件。该策略适用于 IMCM 的组合和并行合成。













































 京公网安备 11010802027423号
京公网安备 11010802027423号