当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iterative Synthesis of Inulin-Type Fructooligosaccharides Enabled by Stereoselective β-d-Fructofuranosylation
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-01 , DOI: 10.1021/acs.joc.2c01849 Liang-Jing Zou 1 , Xing Yang 1 , Xi-Rui Zhao 1 , Huan He 1 , Dan Zhang 1 , Hao Song 1 , Fei Xue 1 , Yong Qin 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-01 , DOI: 10.1021/acs.joc.2c01849 Liang-Jing Zou 1 , Xing Yang 1 , Xi-Rui Zhao 1 , Huan He 1 , Dan Zhang 1 , Hao Song 1 , Fei Xue 1 , Yong Qin 1
Affiliation
|
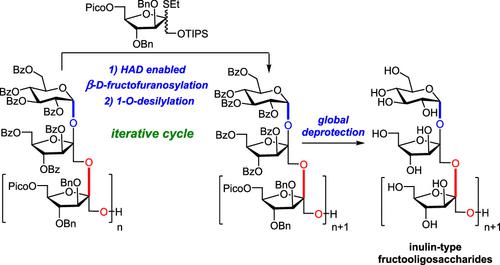
Inulin-type fructooligosaccharides (FOSs) constitute an abundant subgroup of fructans with important biological activities. However, the availability of individual fructooligosaccharides with an accurate structure in high purity and quality remains challenging. We herein report the first iterative synthesis of five inulin-type FOSs with degrees of polymerization ranging from 3 to 7 via highly stereoselective β-(2 → 1)-d-fructofuranosylation on a gram scale. Central to the synthesis is the decisive use of the 1-O-TIPS-6-O-picoloyl-protected fructofuranosyl thioglycoside donor, which assured the excellent β-selective glycosylation by the hydrogen-bond-mediated aglycone delivery (HAD).
中文翻译:

通过立体选择性 β-d-呋喃果糖基化迭代合成菊粉型低聚果糖
菊粉型低聚果糖 (FOS) 构成了丰富的具有重要生物活性的果聚糖亚群。然而,获得具有准确结构的高纯度和高质量的低聚果糖仍然具有挑战性。我们在此报道了通过高度立体选择性 β-(2 → 1) -d-呋喃果糖基化在克尺度上首次迭代合成聚合度为 3 到 7 的五种菊糖型 FOS 。合成的核心是决定性地使用 1- O -TIPS-6- O -picoloyl-protected fructofuranosyl thioglycoside 供体,这通过氢键介导的糖苷配基递送 (HAD) 确保了出色的 β-选择性糖基化。
更新日期:2022-11-01
中文翻译:

通过立体选择性 β-d-呋喃果糖基化迭代合成菊粉型低聚果糖
菊粉型低聚果糖 (FOS) 构成了丰富的具有重要生物活性的果聚糖亚群。然而,获得具有准确结构的高纯度和高质量的低聚果糖仍然具有挑战性。我们在此报道了通过高度立体选择性 β-(2 → 1) -d-呋喃果糖基化在克尺度上首次迭代合成聚合度为 3 到 7 的五种菊糖型 FOS 。合成的核心是决定性地使用 1- O -TIPS-6- O -picoloyl-protected fructofuranosyl thioglycoside 供体,这通过氢键介导的糖苷配基递送 (HAD) 确保了出色的 β-选择性糖基化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号