当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low-Temperature CO2-Assisted Lithium–Oxygen Batteries for Improved Stability of Peroxodicarbonate and Excellent Cyclability
ACS Energy Letters ( IF 19.3 ) Pub Date : 2022-10-31 , DOI: 10.1021/acsenergylett.2c01796 Jin-Hyuk Kang 1 , Jiwon Park 1 , Moony Na 1 , Rak Hyeon Choi 1 , Hye Ryung Byon 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2022-10-31 , DOI: 10.1021/acsenergylett.2c01796 Jin-Hyuk Kang 1 , Jiwon Park 1 , Moony Na 1 , Rak Hyeon Choi 1 , Hye Ryung Byon 1
Affiliation
|
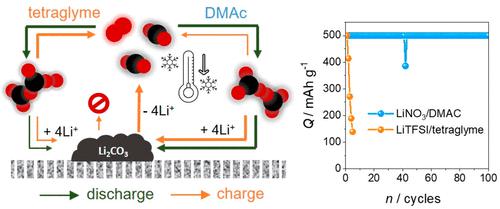
Lithium–oxygen (Li-O2) batteries suffer from undesirable chemical reactions. Side products, such as lithium carbonates (Li2CO3), undergo partial decomposition during charging, and their accumulation leads to poor cyclability. Herein, we show significantly improved cyclability by forming peroxodicarbonate (C2O62–) as the soluble discharging product, hence preserving its stability at low temperatures. The Li-O2 cells comprising 10% CO2 gas and tetraglyme electrolyte solution form anhydride-linked C2O62–. However, despite the improved stability of C2O62– at 0 °C, the low ionic conductivity of tetraglyme results in poor cyclability. In contrast, dimethylacetamide-based cells produce peroxo-linked C2O62– and offer over 100 cycles at −10 °C. During cycles, the first charging plateaus belonging to C2O62– oxidation appear consistently at 3.75 V (vs Li/Li+). In addition, Li2CO3 is entirely decomposed during the second charging plateau at 4.45 V. Our results show promise for the development of highly rechargeable Li-O2/CO2 batteries.
中文翻译:

低温 CO2 辅助锂氧电池可提高过氧化二碳酸盐的稳定性和出色的循环性能
锂氧 (Li-O 2 ) 电池会发生不良化学反应。碳酸锂 (Li 2 CO 3 ) 等副产物在充电过程中会发生部分分解,它们的积累会导致循环性能差。在此,我们通过形成过氧二碳酸盐 (C 2 O 6 2– ) 作为可溶性放电产物,显着提高了循环性能,从而保持其在低温下的稳定性。包含 10% CO 2气体和四甘醇二甲醚电解质溶液的Li-O 2电池形成酸酐连接的 C 2 O 6 2–。然而,尽管 C 2 O的稳定性有所提高6 2–在 0 °C 时,四甘醇二甲醚的低离子电导率导致循环性能差。相比之下,基于二甲基乙酰胺的细胞产生过氧连接的 C 2 O 6 2–并在 -10 °C 下提供超过 100 个循环。在循环期间,属于 C 2 O 6 2–氧化的第一个充电平台始终出现在 3.75 V(相对于 Li/Li +)。此外,Li 2 CO 3在 4.45 V 的第二个充电平台期间完全分解。我们的结果表明有希望开发高度可充电的 Li-O 2 /CO 2电池。
更新日期:2022-10-31
中文翻译:

低温 CO2 辅助锂氧电池可提高过氧化二碳酸盐的稳定性和出色的循环性能
锂氧 (Li-O 2 ) 电池会发生不良化学反应。碳酸锂 (Li 2 CO 3 ) 等副产物在充电过程中会发生部分分解,它们的积累会导致循环性能差。在此,我们通过形成过氧二碳酸盐 (C 2 O 6 2– ) 作为可溶性放电产物,显着提高了循环性能,从而保持其在低温下的稳定性。包含 10% CO 2气体和四甘醇二甲醚电解质溶液的Li-O 2电池形成酸酐连接的 C 2 O 6 2–。然而,尽管 C 2 O的稳定性有所提高6 2–在 0 °C 时,四甘醇二甲醚的低离子电导率导致循环性能差。相比之下,基于二甲基乙酰胺的细胞产生过氧连接的 C 2 O 6 2–并在 -10 °C 下提供超过 100 个循环。在循环期间,属于 C 2 O 6 2–氧化的第一个充电平台始终出现在 3.75 V(相对于 Li/Li +)。此外,Li 2 CO 3在 4.45 V 的第二个充电平台期间完全分解。我们的结果表明有希望开发高度可充电的 Li-O 2 /CO 2电池。































 京公网安备 11010802027423号
京公网安备 11010802027423号