Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2022-10-28 , DOI: 10.1016/j.psep.2022.10.073 Shijin Zhang , Xiaowei Huo , Suzhou Xu , Yanting Zhang , Benyin Zhang , Mingming Wang , Qingguo Wang , Jing Zhang
|
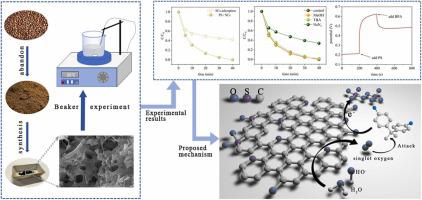
In this work, sulfur-doped (S-doped) carbon materials (SCs) were successfully synthesized by using coffee grounds which are relatively cheap and readily available. Results show that SCs exhibit excellent catalytic activity for persulfate (PS) activation and a more than 99% bisphenol A (BPA) degradation rate was achieved. Meanwhile, 82.3% of total organic carbon (TOC) can be removed in 40 min at 25 °C when applying a dosage of 0.15 g/L SCs and 1 mM PS under an initial pH of about 4.0. SEM and BET characterization methods were used to reveal the surface characteristics of the material. It is found that SCs had a porous structure and a specific surface area of 159.319m2/g, which conferred to SCs superior adsorption (17.3 mg/g). Additionally, kinetic and adsorption isotherm models for adsorption by SCs were obtained via linear fitting, which proved that the process was better described by the pseudo-second-order model (R2 = 0.9989) and Langmuir model (R2 = 0.9890). The free radical quenching experiments, electron paramagnetic resonance (EPR), and open circuit potential (OCP) tests together demonstrated that the system achieved BPA degradation via non-radical pathways (singlet oxygen and electron transfer). It is speculated that S-doping is able to effectively promote electron transfer given the XPS results. Finally, batch control experiments were run to investigate the influence of PS concentration (0.2 – 2.0 mM), BPA concentration (0.005 – 0.03 mM), SCs dose (0.05 – 0.25 g/L), pH (4.0 – 10.0), and Cl− and HCO3− (1 or 5 mM) on the degradation of BPA. Evidently, the catalysts could be used for a wide range of BPA removal scenarios. In a word, S-doped carbon materials prepared from coffee grounds can improve the degradation of BPA and activation of PS under a wide range of operating conditions. Simultaneously it was strongly associated with electron transfer in non-radical pathways. This research lays the foundation for the rational design of a persulfate-based system for use as an actual water purification catalyst.
中文翻译:

咖啡渣合成原硫掺杂碳材料活化过硫酸盐降解BPA:电子转移的关键作用
在这项工作中,使用相对便宜且容易获得的咖啡渣成功地合成了硫掺杂(S掺杂)碳材料(SCs)。结果表明,SCs 对过硫酸盐 (PS) 活化表现出优异的催化活性,双酚 A (BPA) 降解率超过 99%。同时,在初始 pH 值约为 4.0 下,使用 0.15 g/L SCs 和 1 mM PS 的剂量,在 25 °C 下40 分钟内可去除 82.3% 的总有机碳 (TOC) 。SEM 和 BET 表征方法用于揭示材料的表面特性。发现 SCs 具有多孔结构,比表面积为 159.319m 2 /g,这赋予 SCs 优异的吸附性(17.3 毫克/克)。此外,通过线性拟合获得了SCs吸附的动力学和吸附等温线模型,证明伪二级模型(R 2 = 0.9989)和Langmuir模型(R 2 = 0.9890)更好地描述了该过程。自由基猝灭实验、电子顺磁共振 (EPR) 和开路电位 (OCP) 测试共同表明,该系统通过非自由基途径(单线态氧和电子转移)实现了 BPA 降解。鉴于 XPS 结果,推测 S 掺杂能够有效地促进电子转移。最后,进行批次控制实验以研究 PS 浓度 (0.2 – 2.0 mM)、BPA 浓度 (0.005 – 0.03 mM)、SCs 剂量 (0.05 – 0.25)的影响 g/L)、pH (4.0 – 10.0) 和 Cl -和 HCO 3 - (1 或 5 mM) 对 BPA 降解的影响。显然,催化剂可用于广泛的 BPA 去除方案。总之,从咖啡渣制备的S掺杂碳材料可以在广泛的操作条件下改善BPA的降解和PS的活化。同时,它与非自由基途径中的电子转移密切相关。该研究为合理设计基于过硫酸盐的系统用作实际的水净化催化剂奠定了基础。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号