Synlett ( IF 1.7 ) Pub Date : 2022-10-28 , DOI: 10.1055/s-0042-1751378 Kapil Kumar Goel 1, 2 , Rajeev Kharb 2 , Satyendra Kumar Rajput 1 , Prince Prashant Sharma 1 , Monalisa Mukherjee 3
|
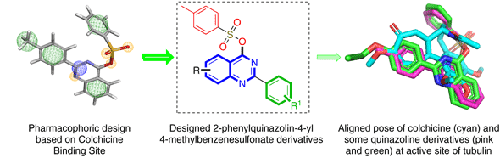
Malignant behavior and multiple abnormal cellular functions have rendered cancer a great challenge for scientists to treat. The rising death toll presents an alarming situation, and the side effects associated with marketed drugs has further increased the quest to develop new anticancer drug molecules. We herein report a rationally designed 2,4-disubstituted quinazoline-based bioactive pharmacophore possessing different substitution patterns to obtain potent anticancer active agents targeting tubulin polymerization. In this series, two compounds showed potent cytotoxicity against all four cancer cell lines (MCF-7, MD-MBA-231, A549, and HCT-116) comparable to that of colchicine. The compounds showed cell cycle arrest in the G2/M phase and induced apoptosis, which showed these compounds might act via binding to the colchicine binding site. These results were further confirmed via tubulin polymerization inhibition, which showed a similar profile to colchicine. Compounds with a propargyl moiety showed very low cytotoxicity as compared to colchicines, even in the presence of a trimethoxy substituent at the quinazoline ring, except for compound case. Two compounds are obtained as potential lead compounds for the development of active anticancer agents, with one having a similar profile to colchicine activity on tubulin polymerization inhibition. These compounds represent promising leads that deserve further investigation and optimization.
中文翻译:

2-Phenylquinazolin-4-yl 4-Methylbenzosulfonate 衍生物通过微管蛋白抑制作为抗癌剂的设计、合成和生物学评价
恶性行为和多种异常细胞功能使癌症成为科学家治疗的巨大挑战。不断上升的死亡人数呈现出令人担忧的情况,与上市药物相关的副作用进一步增加了开发新抗癌药物分子的需求。我们在此报告了一种合理设计的基于 2,4-二取代喹唑啉的生物活性药效团,该药效团具有不同的取代模式,以获得针对微管蛋白聚合的有效抗癌活性剂。在该系列中,两种化合物对所有四种癌细胞系(MCF-7、MD-MBA-231、A549 和 HCT-116)显示出与秋水仙碱相当的强大细胞毒性。这些化合物在 G2/M 期显示细胞周期停滞并诱导细胞凋亡,这表明这些化合物可能通过与秋水仙碱结合位点结合而发挥作用。这些结果通过微管蛋白聚合抑制得到进一步证实,其显示出与秋水仙碱相似的特征。与秋水仙素相比,具有炔丙基部分的化合物显示出非常低的细胞毒性,即使在喹唑啉环上存在三甲氧基取代基的情况下,化合物情况除外。获得了两种化合物作为开发活性抗癌剂的潜在先导化合物,其中一种具有与秋水仙碱相似的微管蛋白聚合抑制活性。这些化合物代表了值得进一步研究和优化的有希望的线索。即使在喹唑啉环上存在三甲氧基取代基的情况下,除了复合情况。获得了两种化合物作为开发活性抗癌剂的潜在先导化合物,其中一种具有与秋水仙碱相似的微管蛋白聚合抑制活性。这些化合物代表了值得进一步研究和优化的有希望的线索。即使在喹唑啉环上存在三甲氧基取代基的情况下,除了复合情况。获得了两种化合物作为开发活性抗癌剂的潜在先导化合物,其中一种具有与秋水仙碱相似的微管蛋白聚合抑制活性。这些化合物代表了值得进一步研究和优化的有希望的线索。







































 京公网安备 11010802027423号
京公网安备 11010802027423号