Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrothermal synthesis of chiral carbon dots
Chirality ( IF 2.8 ) Pub Date : 2022-10-27 , DOI: 10.1002/chir.23509 Anastasia Visheratina 1 , Leila Hesami 2 , Ashleigh K Wilson 2 , Nicole Baalbaki 1 , Natalia Noginova 2 , Mikhail A Noginov 2 , Nicholas A Kotov 1
Chirality ( IF 2.8 ) Pub Date : 2022-10-27 , DOI: 10.1002/chir.23509 Anastasia Visheratina 1 , Leila Hesami 2 , Ashleigh K Wilson 2 , Nicole Baalbaki 1 , Natalia Noginova 2 , Mikhail A Noginov 2 , Nicholas A Kotov 1
Affiliation
|
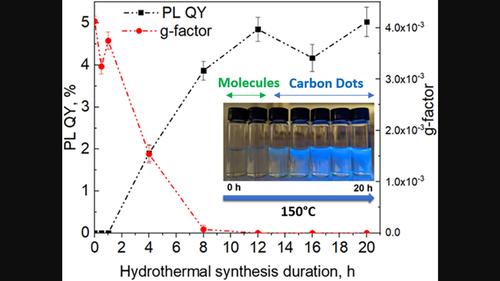
Nanocolloids that are cumulatively referred to as nanocarbons, attracted significant attention during the last decade because of facile synthesis methods, water solubility, tunable photoluminescence, easy surface modification, and high biocompatibility. Among the latest development in this reserach area are chiral nanocarbons exemplified by chiral carbon dots (CDots). They are expected to have applications in sensing, catalysis, imaging, and nanomedicine. However, the current methods of CDots synthesis show often contradictory chemical/optical properties and structural information that required a systematic study with careful structural evaluation. Here, we investigate and optimize chiroptical activity and photoluminescence of L- and D-CDots obtained by hydrothermal carbonization of L- and D-cysteine, respectively. Nuclear magnetic resonance spectroscopy demonstrates that they are formed via gradual dehydrogenation and condensation reactions of the starting amino acid leading to particles with a wide spectrum of functional groups including aromatic cycles. We found that the chiroptical activity of CDots has an inverse correlation with the synthesis duration and temperature, whereas the photoluminescence intensity has a direct one, which is associated with degree of carbonization. Also, our studies show that the hydrothermal synthesis of cysteine in the presence of boric acid leads to the formation of CDots rather than boron nitride nanoparticles as was previously proposed in several reports. These results can be used to design chiral carbon-based nanoparticles with optimal chemical, chiroptical, and photoluminescent properties.
中文翻译:

手性碳点的水热合成
纳米胶体统称为纳米碳,在过去十年中因其简便的合成方法、水溶性、可调谐的光致发光、易于表面改性和高生物相容性而受到广泛关注。该研究领域的最新进展包括手性纳米碳,例如手性碳点 (CDot)。它们有望在传感、催化、成像和纳米医学中得到应用。然而,目前的 CDots 合成方法往往显示出相互矛盾的化学/光学特性和结构信息,需要通过仔细的结构评估进行系统研究。在这里,我们研究并优化了通过水热碳化获得的L-和D -CDot的手性光学活性和光致发光。L-和D-半胱氨酸,分别。核磁共振波谱表明,它们是通过起始氨基酸的逐渐脱氢和缩合反应形成的,从而形成具有包括芳香环在内的广泛官能团的颗粒。我们发现CDots的手性活性与合成持续时间和温度呈负相关,而光致发光强度与碳化程度呈正相关。此外,我们的研究表明,在存在硼酸的情况下,半胱氨酸的水热合成导致形成 CDots,而不是之前在几份报告中提出的氮化硼纳米颗粒。这些结果可用于设计具有最佳化学、手性光学和光致发光特性的手性碳基纳米粒子。
更新日期:2022-10-27
中文翻译:

手性碳点的水热合成
纳米胶体统称为纳米碳,在过去十年中因其简便的合成方法、水溶性、可调谐的光致发光、易于表面改性和高生物相容性而受到广泛关注。该研究领域的最新进展包括手性纳米碳,例如手性碳点 (CDot)。它们有望在传感、催化、成像和纳米医学中得到应用。然而,目前的 CDots 合成方法往往显示出相互矛盾的化学/光学特性和结构信息,需要通过仔细的结构评估进行系统研究。在这里,我们研究并优化了通过水热碳化获得的L-和D -CDot的手性光学活性和光致发光。L-和D-半胱氨酸,分别。核磁共振波谱表明,它们是通过起始氨基酸的逐渐脱氢和缩合反应形成的,从而形成具有包括芳香环在内的广泛官能团的颗粒。我们发现CDots的手性活性与合成持续时间和温度呈负相关,而光致发光强度与碳化程度呈正相关。此外,我们的研究表明,在存在硼酸的情况下,半胱氨酸的水热合成导致形成 CDots,而不是之前在几份报告中提出的氮化硼纳米颗粒。这些结果可用于设计具有最佳化学、手性光学和光致发光特性的手性碳基纳米粒子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号