当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ru(II)/Chiral Carboxylic Acid-Catalyzed Asymmetric [4 + 3] Annulation of Sulfoximines with α,β-Unsaturated Ketones
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-10-27 , DOI: 10.1021/acscatal.2c03531 Pu-Fan Qian 1 , Tao Zhou 1 , Jun-Yi Li 1 , Yi-Bo Zhou 1 , Bing-Feng Shi 1, 2, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-10-27 , DOI: 10.1021/acscatal.2c03531 Pu-Fan Qian 1 , Tao Zhou 1 , Jun-Yi Li 1 , Yi-Bo Zhou 1 , Bing-Feng Shi 1, 2, 3
Affiliation
|
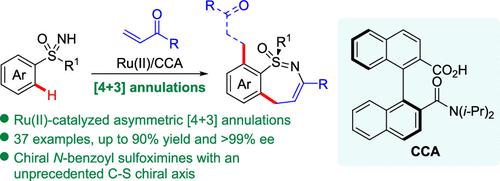
Sulfur-stereogenic containing benzo-fused heterocycles have gained much attention in drug discovery. However, the asymmetric synthesis of these chiral molecules with structural diversity is very challenging. Herein, we report the synthesis of chiral benzothiadiazine-1-oxides with a seven-membered ring via achiral Ru(II)-catalyzed asymmetric [4 + 3] annulation of sulfoximines with α,β-unsaturated ketones assisted by chiral carboxylic acid (CCA). A broad range of chiral benzothiadiazepine-1-oxides bearing various functional groups could be prepared in up to 90% yield with up to >99% ee, expanding the chemical space of chiral sulfoximines. Notably, the oxidative cleavage of the double bonds in the products gave chiral N-benzoyl sulfoximines with a C–S chiral axis.
中文翻译:

Ru(II)/手性羧酸催化的亚砜亚胺与 α,β-不饱和酮的不对称 [4 + 3] 环化反应
含硫立体异构的苯并稠合杂环化合物在药物发现中备受关注。然而,这些具有结构多样性的手性分子的不对称合成非常具有挑战性。在此,我们报道了通过非手性 Ru(II) 催化亚砜亚胺与 α,β-不饱和酮在手性羧酸 (CCA) 辅助下的不对称 [4 + 3] 环化,合成了具有七元环的手性苯并噻二嗪-1-氧化物). 可以以高达 90% 的产率和高达 >99%的ee制备范围广泛的带有各种官能团的手性苯并噻二氮卓-1-氧化物,从而扩展了手性亚砜亚胺的化学空间。值得注意的是,产物中双键的氧化裂解产生了具有 C-S 手性轴的手性N-苯甲酰亚砜亚胺。
更新日期:2022-10-27
中文翻译:

Ru(II)/手性羧酸催化的亚砜亚胺与 α,β-不饱和酮的不对称 [4 + 3] 环化反应
含硫立体异构的苯并稠合杂环化合物在药物发现中备受关注。然而,这些具有结构多样性的手性分子的不对称合成非常具有挑战性。在此,我们报道了通过非手性 Ru(II) 催化亚砜亚胺与 α,β-不饱和酮在手性羧酸 (CCA) 辅助下的不对称 [4 + 3] 环化,合成了具有七元环的手性苯并噻二嗪-1-氧化物). 可以以高达 90% 的产率和高达 >99%的ee制备范围广泛的带有各种官能团的手性苯并噻二氮卓-1-氧化物,从而扩展了手性亚砜亚胺的化学空间。值得注意的是,产物中双键的氧化裂解产生了具有 C-S 手性轴的手性N-苯甲酰亚砜亚胺。















































 京公网安备 11010802027423号
京公网安备 11010802027423号