当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Polysubstituted 2-Oxabicyclo[2.1.1]hexanes via Visible-Light-Induced Energy Transfer
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-10-27 , DOI: 10.1021/jacs.2c09248 Yujie Liang 1 , Roman Kleinmans 1 , Constantin G Daniliuc 1 , Frank Glorius 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-10-27 , DOI: 10.1021/jacs.2c09248 Yujie Liang 1 , Roman Kleinmans 1 , Constantin G Daniliuc 1 , Frank Glorius 1
Affiliation
|
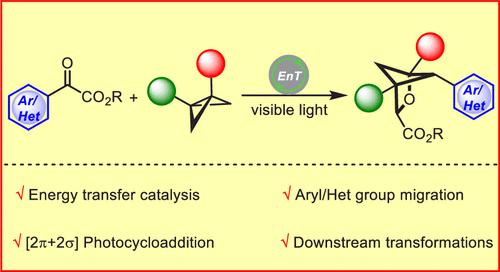
Synthesis of bicyclic scaffolds has attracted growing interest because they are of high importance in modern pharmaceutical development. Here we report a strategy to access polysubstituted 2-oxabicyclo[2.1.1]hexanes in a single operation from readily accessible benzoylformate esters and bicyclo[1.1.0]butanes via visible-light-induced triplet energy transfer catalysis. The process is proposed to involve a formal [2π + 2σ] photocycloaddition/backbone C–H abstraction/aryl group migration sequence. A diverse range of (hetero)aryl groups successfully underwent migration to the backbone (C2) position to provide previously inaccessible bicyclic molecules, and the ester group of the product can serve as a handle for downstream manipulation, thus offering opportunities to rapidly build up molecular complexity and access new sp3-rich chemical space.
中文翻译:

可见光诱导能量转移合成多取代 2-氧杂双环 [2.1.1] 己烷
双环支架的合成引起了越来越多的兴趣,因为它们在现代药物开发中非常重要。在这里,我们报告了一种策略,通过可见光诱导的三重态能量转移催化,从易于获得的苯甲酰甲酸酯和双环 [1.1.0] 丁烷中,在一次操作中获得多取代的 2-氧杂双环 [2.1.1] 己烷。该过程被提议涉及正式的 [2π + 2σ] 光环加成/主链 C-H 抽象/芳基迁移序列。各种各样的(杂)芳基成功地迁移到主链(C2)位置以提供以前无法接近的双环分子,并且产物的酯基可以作为下游操作的手柄,从而提供快速构建分子的机会复杂性和访问新的 sp 3-丰富的化学空间。
更新日期:2022-10-27
中文翻译:

可见光诱导能量转移合成多取代 2-氧杂双环 [2.1.1] 己烷
双环支架的合成引起了越来越多的兴趣,因为它们在现代药物开发中非常重要。在这里,我们报告了一种策略,通过可见光诱导的三重态能量转移催化,从易于获得的苯甲酰甲酸酯和双环 [1.1.0] 丁烷中,在一次操作中获得多取代的 2-氧杂双环 [2.1.1] 己烷。该过程被提议涉及正式的 [2π + 2σ] 光环加成/主链 C-H 抽象/芳基迁移序列。各种各样的(杂)芳基成功地迁移到主链(C2)位置以提供以前无法接近的双环分子,并且产物的酯基可以作为下游操作的手柄,从而提供快速构建分子的机会复杂性和访问新的 sp 3-丰富的化学空间。







































 京公网安备 11010802027423号
京公网安备 11010802027423号