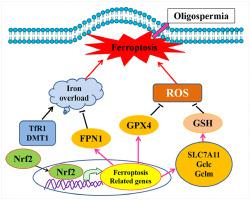
一些少精子症患者精子中核因子-E2 相关因子 2 (Nrf2) 的表达降低。然而,少精症男性精子中 Nrf2 表达减少的机制尚未阐明。在本研究中,我们的临床试验结果表明,与健康男性相比,少精症男性精子中 Nrf2 和谷胱甘肽过氧化物酶 4 (GPX4) 蛋白表达显着降低。在动物实验中,小鼠被随机分为 3 组:野生型 (WT)、Nrf2 敲除组 ( Nrf2 -/- ) 和Nrf2 -/- + 铁死亡抑制剂 (Fer-1) 组。将 Fer-1 腹膜内注射到Nrf2 -/-小鼠中 4 周。结果表明,雄性Nrf2 −/−老鼠表现出精子浓度和活力下降,生育能力显着降低。与 WT 小鼠相比, Nrf2 −/−小鼠睾丸中丙二醛 (MDA) 含量和前列腺素-内过氧化物合酶 2 (Ptgs2) mRNA 表达增加,但烟酰胺腺嘌呤二核苷酸磷酸氧化酶 (NADPH) 含量降低,这是铁死亡的生物标志物。此外,在Nrf2 -/-小鼠中用 Fer-1 治疗可逆转精子浓度和活力的下降。同时,组织学显示生精细胞明显减少, Nrf2 -/-睾丸内形成空泡化。小鼠,通过 Fer-1 治疗逆转。此外,与 WT 小鼠相比,GPX4、溶质载体家族 7 成员 11 (SLC7A11)、谷氨酸-半胱氨酸连接酶、催化亚基 (Gclc)、谷氨酸-半胱氨酸连接酶、修饰亚基 (Gclm) 和铁转运蛋白 1 (FPN1) 的 mRNA 和蛋白表达Nrf2 -/-小鼠的睾丸组织中转铁蛋白受体 1 (TfR1) 和二价金属转运蛋白 1 (DMT1) mRNA 和蛋白质表达显着降低,但转铁蛋白受体 1 (TfR1) 和二价金属转运蛋白 1 (DMT1) mRNA 和蛋白质表达增加。用 Fer-1 处理后,只有 Gclc 和 Gclm mRNA 和蛋白质表达增加。综上所述,我们的数据表明,Nrf2 的缺失导致 GPX4 的下调和其他铁死亡相关基因的调节,导致生精细胞发生铁死亡并最终导致少精子症。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Inhibition of ferroptosis attenuates oligospermia in male Nrf2 knockout mice
Nuclear factor-E2-related factor 2 (Nrf2) expression in sperm decreases in some oligospermia patients. However, the mechanism of reduced Nrf2 expression in sperm of oligospermia men is not elucidated. In the present study, our clinical trial results showed that Nrf2 and glutathione peroxidase 4 (GPX4) protein expressions in sperm of oligospermia men significantly decreased than those of healthy men. In animal experiments, mice were randomly divided into 3 groups: wild type (WT), Nrf2 knockout (Nrf2−/−) and Nrf2−/− + ferroptosis inhibitor (Fer-1) groups. Fer-1 was intraperitoneally injected in Nrf2−/− mice for 4 weeks. The results showed that male Nrf2−/− mice displayed decreased sperm concentration and motility, and significantly lower fertility. Compared with WT mice, malondialdehyde (MDA) content and prostaglandin-endoperoxide synthase 2 (Ptgs2) mRNA expression increased, but nicotinamide adenine dinucleotide phosphate oxidase (NADPH) content decreased in the testes of Nrf2−/− mice, which were biomarkers of ferroptosis. Furthermore, treatment with Fer-1 in Nrf2−/− mice reversed the decreased sperm concentration and motility. Meanwhile, histology showed that spermatogenic cells obviously decreased, and vacuolization formed in the testes of Nrf2−/− mice, which were reversed by Fer-1 treatment. Additionally, compared with WT mice, GPX4, solute carrier family 7 member 11 (SLC7A11), glutamate-cysteine ligase, catalytic subunit (Gclc), glutamate-cysteine ligase, modifier subunit (Gclm) and ferroportin 1 (FPN1) mRNA and protein expressions significantly decreased, but transferrin receptor 1 (TfR1) and divalent metal transporter 1 (DMT1) mRNA and protein expressions increased in testicular tissues in Nrf2−/− mice. After treatment with Fer-1, only Gclc and Gclm mRNA and protein expressions increased. Taken together, our data suggested that deletion of Nrf2 leads to downregulation of GPX4 and regulation of other ferroptosis-related genes, resulting in ferroptosis occurrence in spermatogenic cells and ultimately oligospermia.


































 京公网安备 11010802027423号
京公网安备 11010802027423号