当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of Ti3AlC2 Etching for Ti3C2Tx MXene Synthesis
Chemistry of Materials ( IF 7.2 ) Pub Date : 2022-10-25 , DOI: 10.1021/acs.chemmater.2c02194
Mark Anayee 1 , Christopher E. Shuck 1 , Mikhail Shekhirev 1 , Adam Goad 1 , Ruocun Wang 1 , Yury Gogotsi 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2022-10-25 , DOI: 10.1021/acs.chemmater.2c02194
Mark Anayee 1 , Christopher E. Shuck 1 , Mikhail Shekhirev 1 , Adam Goad 1 , Ruocun Wang 1 , Yury Gogotsi 1
Affiliation
|
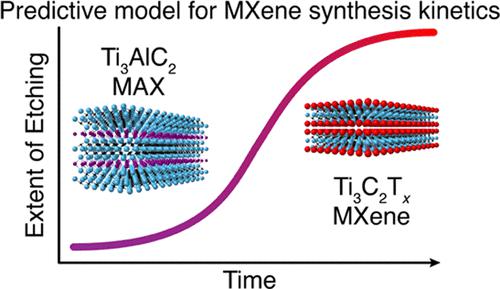
The family of two-dimensional (2D) carbides and nitrides called MXenes has grown to encompass numerous structures and compositions. MXenes have been explored in a variety of applications such as energy storage, wireless communication, optoelectronics, and medicine because of their high electrical conductivity, redox-active surfaces, plasmonic behavior, and other attractive properties. Knowledge of the process kinetics is of fundamental importance for synthesis and property control of MXenes. Prediction of the optimal processing time as a function of various parameters will also facilitate scaling wet chemical synthesis of MXenes for industrial use. Herein, we performed a systematic study of the kinetics of the MAX phase precursor etching reaction for topochemical MXene synthesis by collecting and tracking the evolution of the byproduct H2 gas. For the Ti3AlC2 MAX to Ti3C2Tx MXene conversion, we investigated the influence of critical parameters, such as etchant composition, concentration, temperature, and MAX particle size, on the etching kinetics and developed an empirical predictive model to determine the optimal synthesis conditions given any input parameters. We tested a set of 12 kinetics models as well as a model-free fitting method and found the best agreement with the experimental results from three models and the model-free method (R2 > 0.990). The measured apparent activation energies ranged from 54.2 to 55.7 kJ/mol. Overall, our results suggest NH4HF2 as the most efficient etchant, such that the etching time required to produce Ti3C2Tx can be reduced to a few hours. We also demonstrated the importance of separating MAX powders based on particle size into narrow fractions. Finally, we discuss how this method can be improved and applied to study yet-to-be synthesized MXenes and how the MAX/MXene transformation can serve as a platform to model reactions under confinement.
中文翻译:

用于 Ti3C2Tx MXene 合成的 Ti3AlC2 蚀刻动力学
称为 MXenes 的二维 (2D) 碳化物和氮化物家族已经发展到包含多种结构和成分。MXenes 因其高导电性、氧化还原活性表面、等离子体行为和其他吸引人的特性而在各种应用中得到了探索,例如能量存储、无线通信、光电子学和医学。了解过程动力学对于 MXenes 的合成和性能控制至关重要。根据各种参数预测最佳处理时间也将有助于扩大 MXenes 的湿化学合成以供工业使用。在此,我们通过收集和跟踪副产物 H 的演变,对用于拓扑化学 MXene 合成的 MAX 相前体蚀刻反应的动力学进行了系统研究。2气。对于 Ti 3 AlC 2 MAX 到 Ti 3 C 2 T x MXene 的转化,我们研究了蚀刻剂成分、浓度、温度和 MAX 粒径等关键参数对蚀刻动力学的影响,并开发了一个经验预测模型在给定任何输入参数的情况下确定最佳合成条件。我们测试了一组 12 个动力学模型以及一个无模型拟合方法,发现三个模型和无模型方法的实验结果与实验结果最一致(R 2 > 0.990)。测得的表观活化能范围为 54.2 至 55.7 kJ/mol。总的来说,我们的结果表明 NH 4 HF2作为最有效的蚀刻剂,使得产生Ti 3 C 2 T x所需的蚀刻时间可以减少到几个小时。我们还展示了根据粒度将 MAX 粉末分离成窄部分的重要性。最后,我们讨论了如何改进该方法并将其应用于研究尚未合成的 MXenes,以及如何将 MAX/MXene 转换作为模拟受限反应的平台。
更新日期:2022-10-25
中文翻译:

用于 Ti3C2Tx MXene 合成的 Ti3AlC2 蚀刻动力学
称为 MXenes 的二维 (2D) 碳化物和氮化物家族已经发展到包含多种结构和成分。MXenes 因其高导电性、氧化还原活性表面、等离子体行为和其他吸引人的特性而在各种应用中得到了探索,例如能量存储、无线通信、光电子学和医学。了解过程动力学对于 MXenes 的合成和性能控制至关重要。根据各种参数预测最佳处理时间也将有助于扩大 MXenes 的湿化学合成以供工业使用。在此,我们通过收集和跟踪副产物 H 的演变,对用于拓扑化学 MXene 合成的 MAX 相前体蚀刻反应的动力学进行了系统研究。2气。对于 Ti 3 AlC 2 MAX 到 Ti 3 C 2 T x MXene 的转化,我们研究了蚀刻剂成分、浓度、温度和 MAX 粒径等关键参数对蚀刻动力学的影响,并开发了一个经验预测模型在给定任何输入参数的情况下确定最佳合成条件。我们测试了一组 12 个动力学模型以及一个无模型拟合方法,发现三个模型和无模型方法的实验结果与实验结果最一致(R 2 > 0.990)。测得的表观活化能范围为 54.2 至 55.7 kJ/mol。总的来说,我们的结果表明 NH 4 HF2作为最有效的蚀刻剂,使得产生Ti 3 C 2 T x所需的蚀刻时间可以减少到几个小时。我们还展示了根据粒度将 MAX 粉末分离成窄部分的重要性。最后,我们讨论了如何改进该方法并将其应用于研究尚未合成的 MXenes,以及如何将 MAX/MXene 转换作为模拟受限反应的平台。

































 京公网安备 11010802027423号
京公网安备 11010802027423号