当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Bonding Nature of Fe–CO Complexes in Heme Proteins
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-10-25 , DOI: 10.1021/acs.inorgchem.2c02387 Shuyang Liu 1 , Songyan Xia 1 , Dongxiao Yue 1 , Haoran Sun 1 , Hajime Hirao 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-10-25 , DOI: 10.1021/acs.inorgchem.2c02387 Shuyang Liu 1 , Songyan Xia 1 , Dongxiao Yue 1 , Haoran Sun 1 , Hajime Hirao 1
Affiliation
|
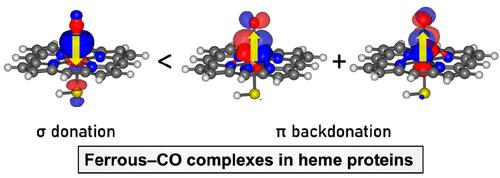
Although carbon monoxide (CO) has been known to bind to the ferrous heme in cytochrome P450 enzymes (P450s) since the earliest days of P450 research, details on the nature of the ferrous–CO bonding remain elusive. This study employed dispersion-corrected density functional theory (DFT) calculations and DFT-based theoretical analyses to investigate the complexes between CO and a thiolate- or imidazole-ligated heme that contains ferric or ferrous iron. Traditionally, the ferrous–CO bonding in heme systems has been interpreted qualitatively in terms of σ donation and π backdonation. Complementary occupied-virtual orbital pair (COVP) analysis yielded one orbital pair for σ donation and two for π backdonation together with the specific magnitude of their energetic contributions. The charge-transfer effect for these three orbital pairs has nearly the same energetic significance in the ferrous–CO complexes. Therefore, in total, the π-backdonation effect is much greater than the σ-donation effect. In contrast, the σ-donation effect is more significant in the ferric–CO complex because of the less efficient π backdonation. The nature of ferric–CO and ferrous–CO bonding was further scrutinized using the generalized Kohn–Sham energy decomposition analysis (GKS-EDA) scheme, whose results highlighted the significance of various effects in enhancing the Fe–CO bonding for the thiolate- and imidazole-ligated heme groups. In particular, the intrinsic repulsion effect plays a crucial role in promoting the preferential binding of CO toward the ferrous heme and in determining the geometry of the complexes.
中文翻译:

血红素蛋白中 Fe-CO 复合物的键合性质
尽管从 P450 研究的早期开始,就已知一氧化碳 (CO) 与细胞色素 P450 酶 (P450s) 中的亚铁血红素结合,但关于亚铁-CO 键合性质的细节仍然难以捉摸。本研究采用色散校正密度泛函理论 (DFT) 计算和基于 DFT 的理论分析来研究 CO 与含有三价铁或亚铁的硫醇盐或咪唑配体血红素之间的复合物。传统上,血红素系统中的亚铁-CO 键已被定性地解释为 σ 捐赠和 π 回馈。互补占据-虚拟轨道对 (COVP) 分析产生了一个用于 σ 捐赠的轨道对和两个用于 π 回馈的轨道对,以及它们的能量贡献的特定大小。这三个轨道对的电荷转移效应在亚铁-CO 配合物中具有几乎相同的能量意义。因此,总的来说,π-回馈效应远大于 σ-回馈效应。相比之下,由于 π 回馈效率较低,在铁-CO 络合物中 σ 捐赠效应更为显着。使用广义 Kohn-Sham 能量分解分析 (GKS-EDA) 方案进一步研究了 ferric-CO 和 ferrous-CO 键合的性质,其结果突出了各种效应在增强硫醇盐的 Fe-CO 键合和咪唑连接的血红素基团。特别是,内在排斥效应在促进 CO 优先结合亚铁血红素和确定复合物的几何形状方面起着至关重要的作用。
更新日期:2022-10-25
中文翻译:

血红素蛋白中 Fe-CO 复合物的键合性质
尽管从 P450 研究的早期开始,就已知一氧化碳 (CO) 与细胞色素 P450 酶 (P450s) 中的亚铁血红素结合,但关于亚铁-CO 键合性质的细节仍然难以捉摸。本研究采用色散校正密度泛函理论 (DFT) 计算和基于 DFT 的理论分析来研究 CO 与含有三价铁或亚铁的硫醇盐或咪唑配体血红素之间的复合物。传统上,血红素系统中的亚铁-CO 键已被定性地解释为 σ 捐赠和 π 回馈。互补占据-虚拟轨道对 (COVP) 分析产生了一个用于 σ 捐赠的轨道对和两个用于 π 回馈的轨道对,以及它们的能量贡献的特定大小。这三个轨道对的电荷转移效应在亚铁-CO 配合物中具有几乎相同的能量意义。因此,总的来说,π-回馈效应远大于 σ-回馈效应。相比之下,由于 π 回馈效率较低,在铁-CO 络合物中 σ 捐赠效应更为显着。使用广义 Kohn-Sham 能量分解分析 (GKS-EDA) 方案进一步研究了 ferric-CO 和 ferrous-CO 键合的性质,其结果突出了各种效应在增强硫醇盐的 Fe-CO 键合和咪唑连接的血红素基团。特别是,内在排斥效应在促进 CO 优先结合亚铁血红素和确定复合物的几何形状方面起着至关重要的作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号