当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analysis of the Mechanism of Polybutadiene Synthesis in the Presence of the Neodymium Versatate + Diisobutylaluminum Hydride + Ethylaluminum Sesquichloride Catalytic System within the Solution of the Inverse Kinetic Problem
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-10-24 , DOI: 10.1021/acs.iecr.2c02960
Konstantin A. Tereshchenko 1 , Nikolai V. Ulitin 1 , Polina S. Bedrina 2 , Daria A. Shiyan 1 , Alexandr D. Lifanov 1 , Timur I. Madzhidov 2 , Svetlana N. Rusanova 1 , Svetoslav I. Volfson 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-10-24 , DOI: 10.1021/acs.iecr.2c02960
Konstantin A. Tereshchenko 1 , Nikolai V. Ulitin 1 , Polina S. Bedrina 2 , Daria A. Shiyan 1 , Alexandr D. Lifanov 1 , Timur I. Madzhidov 2 , Svetlana N. Rusanova 1 , Svetoslav I. Volfson 1
Affiliation
|
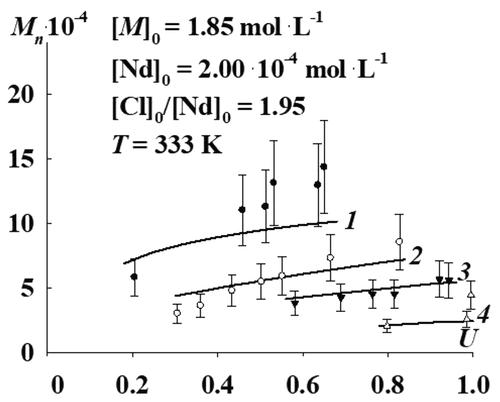
A kinetic model is proposed for polybutadiene synthesis in the presence of the neodymium versatate + diisobutylaluminum hydride + ethylaluminum sesquichloride (NdV + DIBAH + EASC) catalytic system. As a result of solving the inverse kinetic problem, the rate coefficients of reactions were determined. At a quantitative level, it has been shown that polybutadiene synthesis in the presence of the NdV + DIBAH + EASC catalytic system can run according to the scheme, which includes only the chain propagation, chain termination, chain transfer to butadiene, and DIBAH. This fact testifies against the need to involve the reactions of the coordinative chain transfer polymerization mechanism to describe the kinetic regularities of the process in question. Based on the bimodal form of the experimental molar mass distributions of polybutadiene, it was taken into account in the proposed kinetic model that all reactions run on active sites of two types. As a result of the calculation of the characteristic times of chain limitation (in which the chain limitation is a sum of chain termination and transfer rates) on active sites of the first and second types, it was shown that the active sites of the first type are the active sites of classical coordination polymerization (the characteristic time of chain limitation is 3.35 × 101–8.82 × 101 s) and on active sites of the second type, living chain propagation runs. This is due to the fact that the characteristic chain limitation time for active sites of the second type is 9.09 × 103 to 1.35 × 105 s, which is comparable to the time of polybutadiene synthesis until a high butadiene conversion is reached (1.00 × 104 s).
中文翻译:

反动力学问题解法中戊二酸钕+氢化二异丁基铝+倍半氯化乙基铝催化体系合成聚丁二烯的机理分析
提出了在叔碳酸钕 + 二异丁基氢化铝 + 乙基倍半氯化铝 (NdV + DIBAH + EASC) 催化体系存在下合成聚丁二烯的动力学模型。作为解决逆动力学问题的结果,确定了反应的速率系数。在定量水平上,已经表明在 NdV + DIBAH + EASC 催化体系存在下的聚丁二烯合成可以按照该方案进行,该方案仅包括链增长、链终止、链转移至丁二烯和 DIBAH。这一事实证明不需要涉及配位链转移聚合机制的反应来描述所讨论过程的动力学规律。基于聚丁二烯的实验摩尔质量分布的双峰形式,在提议的动力学模型中考虑到所有反应都在两种类型的活性位点上进行。通过计算第一类和第二类活性位点的链限特征时间(其中链限是链终止率和转移率之和),结果表明,第一类活性位点是经典配位聚合的活性位点(链限特征时间为 3.35 × 101 –8.82 × 10 1 s)和第二类活性位点,活链传播运行。这是因为第二类活性位点的特征链限制时间为 9.09 × 10 3到 1.35 × 10 5 s,这与聚丁二烯合成直到达到高丁二烯转化率的时间(1.00 × 10 4秒)。
更新日期:2022-10-24
中文翻译:

反动力学问题解法中戊二酸钕+氢化二异丁基铝+倍半氯化乙基铝催化体系合成聚丁二烯的机理分析
提出了在叔碳酸钕 + 二异丁基氢化铝 + 乙基倍半氯化铝 (NdV + DIBAH + EASC) 催化体系存在下合成聚丁二烯的动力学模型。作为解决逆动力学问题的结果,确定了反应的速率系数。在定量水平上,已经表明在 NdV + DIBAH + EASC 催化体系存在下的聚丁二烯合成可以按照该方案进行,该方案仅包括链增长、链终止、链转移至丁二烯和 DIBAH。这一事实证明不需要涉及配位链转移聚合机制的反应来描述所讨论过程的动力学规律。基于聚丁二烯的实验摩尔质量分布的双峰形式,在提议的动力学模型中考虑到所有反应都在两种类型的活性位点上进行。通过计算第一类和第二类活性位点的链限特征时间(其中链限是链终止率和转移率之和),结果表明,第一类活性位点是经典配位聚合的活性位点(链限特征时间为 3.35 × 101 –8.82 × 10 1 s)和第二类活性位点,活链传播运行。这是因为第二类活性位点的特征链限制时间为 9.09 × 10 3到 1.35 × 10 5 s,这与聚丁二烯合成直到达到高丁二烯转化率的时间(1.00 × 10 4秒)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号