当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overcoming Preclinical Safety Obstacles to Discover (S)-N-((1,2,3,5,6,7-Hexahydro-s-indacen-4-yl)carbamoyl)-6-(methylamino)-6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-sulfonamide (GDC-2394): A Potent and Selective NLRP3 Inhibitor
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-10-24 , DOI: 10.1021/acs.jmedchem.2c01250 Christopher McBride 1 , Lynnie Trzoss 1 , Davide Povero 1 , Milos Lazic 1 , Geza Ambrus-Aikelin 1 , Angelina Santini 1 , Rama Pranadinata 1 , Gretchen Bain 1 , Ryan Stansfield 1 , Jeffrey A Stafford 1 , James Veal 1 , Ryan Takahashi 2 , Justin Ly 2 , Shu Chen 2 , Liling Liu 2 , Marika Nespi 2 , Robert Blake 2 , Arna Katewa 2 , Tracy Kleinheinz 2 , Swathi Sujatha-Bhaskar 2 , Nandhini Ramamoorthi 2 , Jessica Sims 2 , Brent McKenzie 2 , Mark Chen 2 , Mark Ultsch 2 , Matthew Johnson 2 , Jeremy Murray 2 , Claudio Ciferri 2 , Steven T Staben 2 , Michael J Townsend 2 , Craig E Stivala 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-10-24 , DOI: 10.1021/acs.jmedchem.2c01250 Christopher McBride 1 , Lynnie Trzoss 1 , Davide Povero 1 , Milos Lazic 1 , Geza Ambrus-Aikelin 1 , Angelina Santini 1 , Rama Pranadinata 1 , Gretchen Bain 1 , Ryan Stansfield 1 , Jeffrey A Stafford 1 , James Veal 1 , Ryan Takahashi 2 , Justin Ly 2 , Shu Chen 2 , Liling Liu 2 , Marika Nespi 2 , Robert Blake 2 , Arna Katewa 2 , Tracy Kleinheinz 2 , Swathi Sujatha-Bhaskar 2 , Nandhini Ramamoorthi 2 , Jessica Sims 2 , Brent McKenzie 2 , Mark Chen 2 , Mark Ultsch 2 , Matthew Johnson 2 , Jeremy Murray 2 , Claudio Ciferri 2 , Steven T Staben 2 , Michael J Townsend 2 , Craig E Stivala 2
Affiliation
|
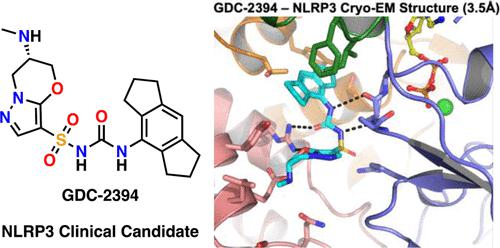
Inappropriate activation of the NLRP3 inflammasome has been implicated in multiple inflammatory and autoimmune diseases. Herein, we aimed to develop novel NLRP3 inhibitors that could minimize the risk of drug-induced liver injury. Lipophilic ligand efficiency was used as a guiding metric to identify a series of 6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazinesulfonylureas. A leading compound from this series was advanced into safety studies in cynomolgus monkeys, and renal toxicity, due to compound precipitation, was observed. To overcome this obstacle, we focused on improving the solubility of our compounds, specifically by introducing basic amine substituents into the scaffold. This led to the identification of GDC-2394, a potent and selective NLRP3 inhibitor, with an in vitro and in vivo safety profile suitable for advancement into human clinical trials.
中文翻译:

克服发现 (S)-N-((1,2,3,5,6,7-Hexahydro-s-indacen-4-yl)carbamoyl)-6-(methylamino)-6,7-dihydro 的临床前安全障碍-5H-吡唑并[5,1-b][1,3]oxazine-3-sulfonamide (GDC-2394):一种有效且选择性的 NLRP3 抑制剂
NLRP3 炎性体的不适当激活与多种炎症和自身免疫疾病有关。在此,我们的目标是开发新的 NLRP3 抑制剂,以最大限度地降低药物性肝损伤的风险。亲脂性配体效率被用作识别一系列 6,7-二氢-5H-吡唑并[5,1- b][1,3]恶嗪磺酰脲类。该系列中的一种领先化合物已进入食蟹猴的安全性研究,并观察到由于化合物沉淀导致的肾毒性。为了克服这个障碍,我们专注于提高化合物的溶解度,特别是通过在支架中引入碱性胺取代基。这导致了 GDC-2394 的鉴定,这是一种有效的选择性 NLRP3 抑制剂,具有适合推进人体临床试验的体外和体内安全性特征。
更新日期:2022-10-24
中文翻译:

克服发现 (S)-N-((1,2,3,5,6,7-Hexahydro-s-indacen-4-yl)carbamoyl)-6-(methylamino)-6,7-dihydro 的临床前安全障碍-5H-吡唑并[5,1-b][1,3]oxazine-3-sulfonamide (GDC-2394):一种有效且选择性的 NLRP3 抑制剂
NLRP3 炎性体的不适当激活与多种炎症和自身免疫疾病有关。在此,我们的目标是开发新的 NLRP3 抑制剂,以最大限度地降低药物性肝损伤的风险。亲脂性配体效率被用作识别一系列 6,7-二氢-5H-吡唑并[5,1- b][1,3]恶嗪磺酰脲类。该系列中的一种领先化合物已进入食蟹猴的安全性研究,并观察到由于化合物沉淀导致的肾毒性。为了克服这个障碍,我们专注于提高化合物的溶解度,特别是通过在支架中引入碱性胺取代基。这导致了 GDC-2394 的鉴定,这是一种有效的选择性 NLRP3 抑制剂,具有适合推进人体临床试验的体外和体内安全性特征。






























 京公网安备 11010802027423号
京公网安备 11010802027423号