Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitating α-amidated peptide degradation by separative technologies and ultra-high resolution mass spectrometry
Talanta ( IF 5.6 ) Pub Date : 2022-10-23 , DOI: 10.1016/j.talanta.2022.124036 Elodie Logerot , Catherine Perrin , Yoann Ladner , Frédéric Aubriet , Vincent Carré , Christine Enjalbal
Talanta ( IF 5.6 ) Pub Date : 2022-10-23 , DOI: 10.1016/j.talanta.2022.124036 Elodie Logerot , Catherine Perrin , Yoann Ladner , Frédéric Aubriet , Vincent Carré , Christine Enjalbal
|
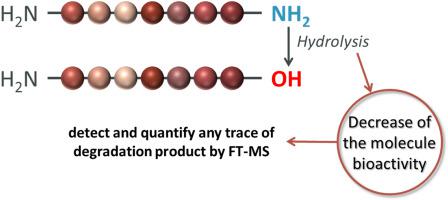
α-amidation of peptides is a C -terminal modification allowing to improve half-life of therapeutic peptides. However, due to storage conditions, this C -terminal amide function can be partially hydrolyzed into a carboxylic acid moiety, resulting in a decrease of the molecule bioactivity. It is therefore necessary to be able to detect and quantify any trace of this degradation product when dealing with such class of peptides. For this purpose, LC-UV, CE-UV, LC/MS and LC/MS/MS are conventional analytical technologies used in pharmaceutical laboratories to monitor peptide integrity. Since the production of the carboxylated peptide issued from the amidated starting compound is scrutinized, ultra-high-resolution mass spectrometry was investigated without the recourse to any separative method (direct infusion mode) to achieve rapid straightforward relative quantitative analyses from the detected isotopic clusters of both peptides. Indeed, low or high-resolution MS instruments present in pharmaceutical laboratories (fitted with Q, IT or ToF mass analyzers) allow to distinguish both molecular ions that differ from 0.984 Da (NH2 formally substituted by OH). However, the analytical difficulty comes from the fact that the A+1 ion (13 C contribution) of the amidated peptide and the A ion (12 C contribution) of any produced carboxylated sequence are overlapping hampering the detection of minute amount of the hydrolyzed product. Actually, to differentiate these two isobaric ions, mass analyzers should possess at least a resolution of 90 000 for 7 residues peptides (molecular ions around 700–800 Da) which is presumably attainable with any FT-MS instrument. With an ultra-high-resolution mass spectrometer (FT-ICR MS) the observed fine isotopic structure displaying all expected isotopologues permitted in a single fast analysis to unambiguously detect both compounds (non-hydrolyzed and hydrolyzed one) and subsequently define the relative amount of the carboxylated peptide contaminant. Finally, these results were confronted with conventional measurements recorded with orthogonal methodologies such as LC-UV and CE-UV. The pro and cons of all quantitative strategies are discussed.
中文翻译:

通过分离技术和超高分辨率质谱定量 α-酰胺化肽降解
肽的 α-酰胺化是一种 C 端修饰,可以改善治疗性肽的半衰期。然而,由于储存条件的原因,这种 C 端酰胺官能团可以部分水解成羧酸部分,导致分子生物活性降低。因此,在处理此类肽时,必须能够检测和定量这种降解产物的任何痕量。为此,LC-UV、CE-UV、LC/MS 和 LC/MS/MS 是制药实验室中用于监测肽完整性的常规分析技术。由于对酰胺化起始化合物产生的羧化肽的生产进行了仔细检查,因此研究了超高分辨率质谱法,而无需求助于任何分离方法(直接输注模式),以便从检测到的两种肽的同位素簇中实现快速、直接的相对定量分析。事实上,制药实验室中的低分辨率或高分辨率 MS 仪器(配备 Q、IT 或 ToF 质量分析仪)可以区分不同于 0.984 Da 的两种分子离子(NH2 正式被 OH 取代)。然而,分析困难在于,酰胺化肽的 A+1 离子(13C 贡献)和任何产生的羧化序列的 A 离子(12C 贡献)重叠,阻碍了微量水解产物的检测。实际上,为了区分这两种同量异位离子,质量分析仪对 7 个残基肽(分子离子约为 700-800 Da)的分辨率至少应为 90 000,这可能是任何 FT-MS 仪器都可以达到的。 使用超高分辨率质谱仪 (FT-ICR MS) 观察到的精细同位素结构显示了单次快速分析中允许的所有预期同位素异构体,以明确检测两种化合物(非水解和水解化合物),并随后确定羧化肽污染物的相对量。最后,这些结果面对使用正交方法(如 LC-UV 和 CE-UV)记录的常规测量。讨论了所有量化策略的优缺点。
更新日期:2022-10-23
中文翻译:

通过分离技术和超高分辨率质谱定量 α-酰胺化肽降解
肽的 α-酰胺化是一种 C 端修饰,可以改善治疗性肽的半衰期。然而,由于储存条件的原因,这种 C 端酰胺官能团可以部分水解成羧酸部分,导致分子生物活性降低。因此,在处理此类肽时,必须能够检测和定量这种降解产物的任何痕量。为此,LC-UV、CE-UV、LC/MS 和 LC/MS/MS 是制药实验室中用于监测肽完整性的常规分析技术。由于对酰胺化起始化合物产生的羧化肽的生产进行了仔细检查,因此研究了超高分辨率质谱法,而无需求助于任何分离方法(直接输注模式),以便从检测到的两种肽的同位素簇中实现快速、直接的相对定量分析。事实上,制药实验室中的低分辨率或高分辨率 MS 仪器(配备 Q、IT 或 ToF 质量分析仪)可以区分不同于 0.984 Da 的两种分子离子(NH2 正式被 OH 取代)。然而,分析困难在于,酰胺化肽的 A+1 离子(13C 贡献)和任何产生的羧化序列的 A 离子(12C 贡献)重叠,阻碍了微量水解产物的检测。实际上,为了区分这两种同量异位离子,质量分析仪对 7 个残基肽(分子离子约为 700-800 Da)的分辨率至少应为 90 000,这可能是任何 FT-MS 仪器都可以达到的。 使用超高分辨率质谱仪 (FT-ICR MS) 观察到的精细同位素结构显示了单次快速分析中允许的所有预期同位素异构体,以明确检测两种化合物(非水解和水解化合物),并随后确定羧化肽污染物的相对量。最后,这些结果面对使用正交方法(如 LC-UV 和 CE-UV)记录的常规测量。讨论了所有量化策略的优缺点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号