Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2022-10-22 , DOI: 10.1016/j.cclet.2022.107935 Chenglong Feng , Xin Liu , Yuanbin She , Zhenlu Shen , Meichao Li
|
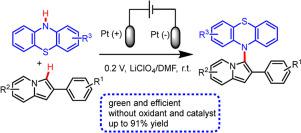
A facile and elegant method for synthesis of novel N–aryl phenothiazine derivatives from 2-phenylindolizines and phenothiazines through direct electrochemical oxidation has been developed. This approach was performed smoothly at room temperature without external oxidant and catalyst. Cyclic voltammetry and in situ FTIR techniques were applied to analyze the cross-coupling process of phenothiazines and 2-phenylindolizines, which helped to select the appropriate reaction potential. Under the optimized conditions, a broad range of substrates were well tolerated, affording the desired products in moderate to excellent isolated yields (up to 91%) with high regioselectivity. Meanwhile, a plausible mechanism involving a radical pathway has been proposed.
中文翻译:

2-苯基吲哚与吩噻嗪电化学C-H/N-H交叉偶联合成新型N-芳基吩噻嗪衍生物
开发了一种通过直接电化学氧化从 2-苯基吲哚和吩噻嗪合成新型N-芳基吩噻嗪衍生物的简便而优雅的方法。该方法在室温下顺利进行,无需外部氧化剂和催化剂。应用循环伏安法和原位FTIR 技术分析了吩噻嗪和 2-苯基吲哚的交叉偶联过程,有助于选择合适的反应电位。在优化的条件下,广泛的底物具有良好的耐受性,以中等到优异的分离产率(高达 91%)和高区域选择性提供所需的产品。同时,已经提出了一种涉及自由基途径的似是而非的机制。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号