Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-10-21 , DOI: 10.1016/j.cej.2022.139914 Tong-fang Jing , Da-xia Zhang , Yan Jin , Guo-dong Si , Bei-xing Li , Wei Mu , Feng Liu
|
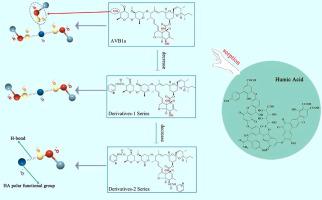
Sorption is the key process that regulates the dispersion of pesticides in the environment once they are introduced into the soil. However, the relationship between the molecular structures of these compounds and the sorption mechanism needs to be further clarified. Our objectives were to uncover sorption drivers in distinct soil samples for the parent avermectin B1a (–OH:3) and pharmacophore-altered derivatives [derivative-1 (–OH:2) and derivative-2 (–OH:1) series]. Avermectin B1a sorption to soil was extensive and essentially irreversible, with avermectin B1a exhibiting significantly greater sorption than the derivatives. The sorption difference between avermectin B1a and derivatives in soil suggested that –OH pharmacophore was the main sorption site for avermectin B1a in soil. Further sorption tests on avermectin B1a and derivatives were carried out on humic acid fractions (HA1 and HA2) that we extracted from the soil. Avermectin B1a sorbed most extensively to humic acid fractions, followed by derivative-1 and derivative-2, thereby indicating the –OH pharmacophore remains the main site for the sorption of avermectin B1a and derivatives by the humic acid fractions. The HA1 fraction showed significantly greater sorption of the compound than the HA2 fraction. Elemental analysis and 13C NMR analysis demonstrated that the HA1 fraction contains more polar functional groups than the HA2 fraction. This suggests that the sorption of avermectin B1a and derivatives by the soil is due to the interaction between the –OH pharmacophore and the polar functional groups in the humic acid, implying that hydrogen bonding may be the main sorption mechanism. This study highlighted the potential sorption mechanisms of pharmacophores in pesticide compounds with humic acid fractions.
中文翻译:

腐植酸性质和化合物结构共同决定了土壤吸附阿维菌素 B1a 及其衍生物的能力
吸附是调节农药在环境中扩散的关键过程,一旦它们被引入土壤。然而,这些化合物的分子结构与吸附机理之间的关系需要进一步阐明。我们的目标是揭示不同土壤样品中母体阿维菌素 B1a (-OH:3) 和药效团改变的衍生物 [derivative-1 (-OH:2) 和衍生物-2 (-OH:1) 系列] 的吸附驱动因素。阿维菌素 B1a 对土壤的吸附是广泛的并且基本上是不可逆的,阿维菌素 B1a 表现出比衍生物显着更大的吸附。阿维菌素B1a与衍生物在土壤中的吸附差异表明-OH药效团是阿维菌素B1a在土壤中的主要吸附位点。对我们从土壤中提取的腐殖酸组分(HA1 和 HA2)进行了对阿维菌素 B1a 和衍生物的进一步吸附试验。阿维菌素 B1a 最广泛地吸附到腐植酸馏分中,其次是衍生物 1 和衍生物 2,因此表明 -OH 药效团仍然是腐植酸馏分吸附阿维菌素 B1a 和衍生物的主要位点。HA1部分显示出比HA2部分显着更大的化合物吸附。元素分析和 HA1部分显示出比HA2部分显着更大的化合物吸附。元素分析和 HA1部分显示出比HA2部分显着更大的化合物吸附。元素分析和13 C NMR分析表明HA1部分比HA2部分含有更多的极性官能团。这表明土壤对阿维菌素 B1a 及其衍生物的吸附是由于 -OH 药效团与腐植酸中的极性官能团之间的相互作用,这意味着氢键可能是主要的吸附机制。本研究强调了农药化合物中药效团与腐植酸组分的潜在吸附机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号