Chemical Data Collections Pub Date : 2022-10-22 , DOI: 10.1016/j.cdc.2022.100962 K.S. Sagar , S. Shamanth , Karthik Kumara , N.K. Lokanath , K. Mantelingu , M.N. Kumara
|
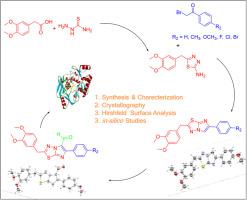
The synthesis of novel imidazo[2,1-b][1,3,4]thiadiazole derivatives through the reaction of 3,4 dimethoxy phenylacetic acid with various 2 bromo acetophenones is been presented. Further all the derivates were performed for formylation reaction for the introduction of the aldehyde functional group at C-4 position of the target molecule. The Structure of these compounds were well characterized by various spectroscopic techniques such as 1H and 13C NMR, mass spectroscopy and FT-IR. X-ray crystallographic study confirmed the structure of imidazothiadiazole and the title compound. The CCDC and ORTEP has been presented. Hirshfeld surface analysis and calculations were carried out to explore intermolecular interactions present in the molecule. The analysis of 2D fingerprint plot gives the quantitative contributions of molecular contacts to the total Hirshfeld surface. in-silico molecular docking studies were performed to check their binding affinities with antimicrobial E. coli MurBenzyme receptor in comparison with the standard ciprofloxacin. Docking scores obtained predicts that two of the novel molecules are potent antimicrobial agents.
中文翻译:

2,6-二取代咪唑噻二唑 5-甲醛:合成、晶体结构解析和计算机研究
介绍了通过3,4-二甲氧基苯乙酸与各种2溴苯乙酮反应合成新型咪唑并[2,1-b][1,3,4]噻二唑衍生物。此外,所有衍生物都进行了甲酰化反应,以在目标分子的C-4位引入醛官能团。这些化合物的结构通过各种光谱技术如1 H 和13 C NMR、质谱和 FT-IR 进行了很好的表征。X 射线晶体学研究证实了咪唑噻二唑和标题化合物的结构。CCDC和ORTEP已提出。进行 Hirshfeld 表面分析和计算以探索分子中存在的分子间相互作用。二维指纹图的分析给出了分子接触对整个 Hirshfeld 表面的定量贡献。与标准环丙沙星相比,进行了计算机分子对接研究以检查它们与抗菌大肠杆菌 MurBenzyme 受体的结合亲和力。获得的对接分数预测其中两种新型分子是有效的抗微生物剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号