当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Biological Evaluation of Novel Pyrazol-5-yl-benzamide Derivatives Containing Oxazole Group as Potential Succinate Dehydrogenase Inhibitors
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-10-21 , DOI: 10.1021/acs.jafc.2c04708 Xiang Cheng 1 , Zonghan Xu 1 , Huisheng Luo 1 , Xihao Chang 1 , Xianhai Lv 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-10-21 , DOI: 10.1021/acs.jafc.2c04708 Xiang Cheng 1 , Zonghan Xu 1 , Huisheng Luo 1 , Xihao Chang 1 , Xianhai Lv 1, 2
Affiliation
|
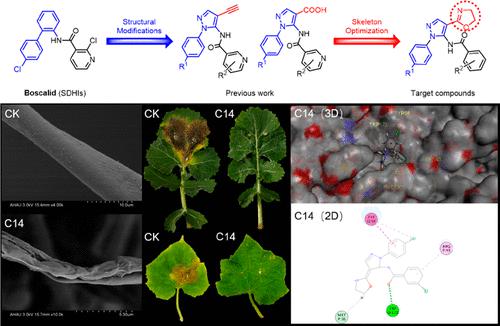
A series of pyrazol-5-yl-benzamide derivatives containing the oxazole group were designed and synthesized as potential SDH inhibitors. According to the results of the bioassays, most target compounds displayed moderate-to-excellent in vitro antifungal activities against Valsa mali, Sclerotinia scleotiorum, Alternaria alternata, and Botrytis cinerea. Among them, compounds C13, C14, and C16 exhibited more excellently inhibitory activities against S. sclerotiorum than boscalid (EC50 = 0.96 mg/L), with EC50 values of 0.69, 0.26, and 0.95 mg/L, respectively. In vivo experiments on rape leaves and cucumber leaves showed that compounds C13 and C14 exhibited considerable protective effects against S. sclerotiorum than boscalid. SEM analysis indicated that compounds C13 and C14 significantly destroyed the typical structure and morphology of S. scleotiorum hyphae. In the respiratory inhibition effect assays, compounds C13 (28.0%) and C14 (33.9%) exhibited a strong inhibitory effect on the respiration rate of S. sclerotiorum mycelia, which was close to boscalid (30.6%). The results of molecular docking indicated that compounds C13 and C14 could form strong interactions with the key residues TRP O:173, ARG P:43, TYR Q:58, and MET P:43 of the SDH. Furthermore, the antifungal mechanism of these derivatives was demonstrated by the SDH enzymatic inhibition assay. These results demonstrate that compounds C13 and C14 can be developed into novel SDH inhibitors for crop protection.
中文翻译:

含有恶唑基团的新型吡唑-5-基-苯甲酰胺衍生物的设计、合成和生物学评价作为潜在的琥珀酸脱氢酶抑制剂
设计并合成了一系列含有恶唑基团的吡唑-5-基苯甲酰胺衍生物作为潜在的 SDH 抑制剂。根据生物测定的结果,大多数目标化合物对马里瓦尔萨、核盘菌、交替链格孢和灰霉病菌表现出中等至优异的体外抗真菌活性。其中,化合物C13、C14和C16对核盘菌的抑制活性比啶酰菌胺(EC 50 = 0.96 mg/L)更优异,EC 50值分别为 0.69、0.26 和 0.95 mg/L。油菜叶和黄瓜叶的体内实验表明,化合物C13和C14对核盘菌的保护作用比啶酰菌胺强。SEM分析表明化合物C13和C14显着破坏了核盘菌菌丝的典型结构和形态。在呼吸抑制作用测定中,化合物C13(28.0%)和C14 (33.9%)对核盘菌菌丝体的呼吸速率有很强的抑制作用,接近于啶酰菌胺(30.6%)。分子对接结果表明化合物C13和C14可以与SDH的关键残基TRP O:173、ARG P:43、TYR Q:58和MET P:43形成强相互作用。此外,这些衍生物的抗真菌机制通过 SDH 酶抑制测定得到证实。这些结果表明,化合物C13和C14可以开发成用于作物保护的新型 SDH 抑制剂。
更新日期:2022-10-21
中文翻译:

含有恶唑基团的新型吡唑-5-基-苯甲酰胺衍生物的设计、合成和生物学评价作为潜在的琥珀酸脱氢酶抑制剂
设计并合成了一系列含有恶唑基团的吡唑-5-基苯甲酰胺衍生物作为潜在的 SDH 抑制剂。根据生物测定的结果,大多数目标化合物对马里瓦尔萨、核盘菌、交替链格孢和灰霉病菌表现出中等至优异的体外抗真菌活性。其中,化合物C13、C14和C16对核盘菌的抑制活性比啶酰菌胺(EC 50 = 0.96 mg/L)更优异,EC 50值分别为 0.69、0.26 和 0.95 mg/L。油菜叶和黄瓜叶的体内实验表明,化合物C13和C14对核盘菌的保护作用比啶酰菌胺强。SEM分析表明化合物C13和C14显着破坏了核盘菌菌丝的典型结构和形态。在呼吸抑制作用测定中,化合物C13(28.0%)和C14 (33.9%)对核盘菌菌丝体的呼吸速率有很强的抑制作用,接近于啶酰菌胺(30.6%)。分子对接结果表明化合物C13和C14可以与SDH的关键残基TRP O:173、ARG P:43、TYR Q:58和MET P:43形成强相互作用。此外,这些衍生物的抗真菌机制通过 SDH 酶抑制测定得到证实。这些结果表明,化合物C13和C14可以开发成用于作物保护的新型 SDH 抑制剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号