Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The RNA repair proteins RtcAB regulate transcription activator RtcR via its CRISPR-associated Rossmann fold domain
iScience ( IF 4.6 ) Pub Date : 2022-10-20 , DOI: 10.1016/j.isci.2022.105425 Ioly Kotta-Loizou 1 , Maria Grazia Giuliano 2 , Milija Jovanovic 1 , Jorrit Schaefer 1 , Fuzhou Ye 3 , Nan Zhang 1, 4 , Danai Athina Irakleidi 1 , Xiaojiao Liu 3, 5 , Xiaodong Zhang 3 , Martin Buck 1 , Christoph Engl 1, 2
iScience ( IF 4.6 ) Pub Date : 2022-10-20 , DOI: 10.1016/j.isci.2022.105425 Ioly Kotta-Loizou 1 , Maria Grazia Giuliano 2 , Milija Jovanovic 1 , Jorrit Schaefer 1 , Fuzhou Ye 3 , Nan Zhang 1, 4 , Danai Athina Irakleidi 1 , Xiaojiao Liu 3, 5 , Xiaodong Zhang 3 , Martin Buck 1 , Christoph Engl 1, 2
Affiliation
|
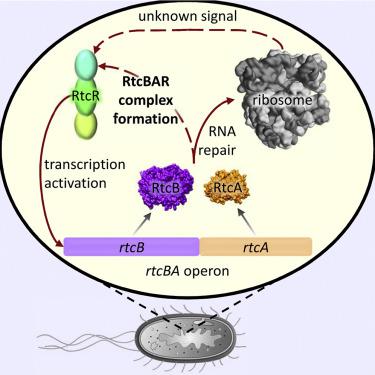
CRISPR-associated Rossmann fold (CARF) domain signaling underpins modulation of CRISPR-Cas nucleases; however, the RtcR CARF domain controls expression of two conserved RNA repair enzymes, cyclase RtcA and ligase RtcB. Here, we demonstrate that RtcAB are required for RtcR-dependent transcription activation and directly bind to RtcR CARF. RtcAB catalytic activity is not required for complex formation with CARF, but is essential yet not sufficient for RtcRAB-dependent transcription activation, implying the need for an additional RNA repair-dependent activating signal. This signal differs from oligoadenylates, a known ligand of CARF domains, and instead appears to originate from the translation apparatus: RtcB repairs a tmRNA that rescues stalled ribosomes and increases translation elongation speed. Taken together, our data provide evidence for an expanded range for CARF domain signaling, including the first evidence of its control via protein-protein interactions, and a feed-forward mechanism to regulate RNA repair required for a functioning translation apparatus.
中文翻译:

RNA 修复蛋白 RtcAB 通过其 CRISPR 相关罗斯曼折叠结构域调节转录激活子 RtcR
CRISPR 相关罗斯曼折叠 (CARF) 结构域信号传导支持 CRISPR-Cas 核酸酶的调节;然而,RtcR CARF 结构域控制两种保守的 RNA 修复酶:环化酶 RtcA 和连接酶 RtcB 的表达。在这里,我们证明 RtcAB 是 RtcR 依赖性转录激活所必需的,并直接与 RtcR CARF 结合。 RtcAB 催化活性对于与 CARF 形成复合物不是必需的,但对于 RtcRAB 依赖性转录激活来说是必要的但还不够,这意味着需要额外的 RNA 修复依赖性激活信号。该信号不同于寡腺苷酸(CARF 结构域的一种已知配体),而是似乎源自翻译装置:RtcB 修复 tmRNA,从而挽救停滞的核糖体并提高翻译延伸速度。总而言之,我们的数据为 CARF 结构域信号传导范围的扩大提供了证据,包括其通过蛋白质-蛋白质相互作用进行控制的第一个证据,以及调节功能性翻译装置所需的 RNA 修复的前馈机制。
更新日期:2022-10-20
中文翻译:

RNA 修复蛋白 RtcAB 通过其 CRISPR 相关罗斯曼折叠结构域调节转录激活子 RtcR
CRISPR 相关罗斯曼折叠 (CARF) 结构域信号传导支持 CRISPR-Cas 核酸酶的调节;然而,RtcR CARF 结构域控制两种保守的 RNA 修复酶:环化酶 RtcA 和连接酶 RtcB 的表达。在这里,我们证明 RtcAB 是 RtcR 依赖性转录激活所必需的,并直接与 RtcR CARF 结合。 RtcAB 催化活性对于与 CARF 形成复合物不是必需的,但对于 RtcRAB 依赖性转录激活来说是必要的但还不够,这意味着需要额外的 RNA 修复依赖性激活信号。该信号不同于寡腺苷酸(CARF 结构域的一种已知配体),而是似乎源自翻译装置:RtcB 修复 tmRNA,从而挽救停滞的核糖体并提高翻译延伸速度。总而言之,我们的数据为 CARF 结构域信号传导范围的扩大提供了证据,包括其通过蛋白质-蛋白质相互作用进行控制的第一个证据,以及调节功能性翻译装置所需的 RNA 修复的前馈机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号