Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2022-10-21 , DOI: 10.1016/j.bioorg.2022.106175 Sumra Malik 1 , G A Miana 1 , Athar Ata 2 , Madiha Kanwal 3 , Saima Maqsood 1 , Imran Malik 4 , Zartashia Kazmi 5
|
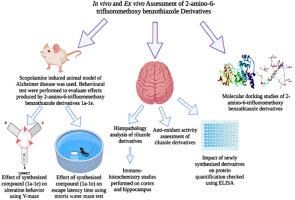
Alzheimer’s disease (AD), a relentless neurodegenerative disorder, is still waiting for safer profile drugs, risk factors affecting AD’s pathogenesis include aβ accumulation, tau protein hyperphosphorylation, and neuroinflammation. This research aimed to synthesize 2-amino-6‑trifluoromethoxy benzothiazole schiff bases. Synthesis was straightforward, combining the riluzole skeleton with compounds containing the azomethine group. Schiff bases synthesized were characterized spectroscopically using proton NMR (1H NMR), and FTIR. In-vivo biological evaluation against scopolamine-induced neuronal damage revealed that these newly synthesized schiff bases were effective in protecting neurons against neuroinflammatory mediators. In-vitro results revealed that these compounds had remarkable potential in improving the anti-oxidant levels. It downregulated glutathione (GSH), glutathione S-transferase (GST), catalase levels, and upregulated lipid peroxidation (LPO) levels. Immunohistochemical studies revealed that groups treated with the newly synthesized schiff bases had reduced expression of inflammatory mediators such as cyclooxygenase 2 (COX-2), JNK, tumor necrosis factor (TNF-α), nuclear factor kappa B (NF-kB) in contrast to the disease group. Moreover, molecular docking studies on these compounds also showed that they possessed a better binding affinity for above mentioned inflammatory mediators. The results of these studies showed that 2-amino-6-trifluoromethoxy benzothiazole schiff bases are remarkably effective against oxidative stress-mediated neuroinflammation.
中文翻译:

新型 2-氨基-6-三氟甲氧基苯并噻唑衍生物的合成、表征、计算机模拟和药理学评价
阿尔茨海默病 (AD) 是一种无情的神经退行性疾病,仍在等待更安全的药物,影响 AD 发病机制的危险因素包括 aβ 积累、tau 蛋白过度磷酸化和神经炎症。本研究旨在合成2-氨基-6-三氟甲氧基苯并噻唑席夫碱。合成很简单,将利鲁唑骨架与含有偶氮甲碱基团的化合物组合在一起。使用质子 NMR ( 1 H NMR) 和 FTIR 对合成的席夫碱进行光谱表征。针对东莨菪碱诱导的神经元损伤的体内生物学评估表明,这些新合成的席夫碱可有效保护神经元免受神经炎症介质的侵害。体外结果表明,这些化合物在提高抗氧化水平方面具有显着潜力。它下调谷胱甘肽 (GSH)、谷胱甘肽 S-转移酶 (GST)、过氧化氢酶水平,并上调脂质过氧化 (LPO) 水平。免疫组织化学研究表明,与新合成的席夫碱治疗组相比,环加氧酶 2 (COX-2)、JNK、肿瘤坏死因子 (TNF-α)、核因子 kappa B (NF-kB) 等炎症介质的表达减少到疾病组。此外,对这些化合物的分子对接研究也表明它们对上述炎症介质具有更好的结合亲和力。这些研究的结果表明,2-amino-6-trifluoromethoxy benzothiazole schiff bases 对氧化应激介导的神经炎症非常有效。







































 京公网安备 11010802027423号
京公网安备 11010802027423号