European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-10-21 , DOI: 10.1016/j.ejmech.2022.114862 Yunong Zhang 1 , Shinpan Chan 1 , Rui He 1 , Yiling Liu 1 , Xiaojuan Song 1 , Zheng-Chao Tu 2 , Xiaomei Ren 3 , Yang Zhou 1 , Zhang Zhang 1 , Zhen Wang 3 , Fengtao Zhou 1 , Ke Ding 4
|
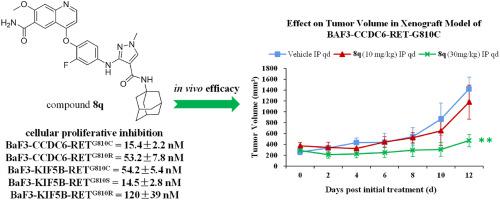
REarranged during Transfection (RET) is a validated target for anticancer drug discovery and two selective RET inhibitors were approved by US FDA in 2020. However, acquired resistance mediated by secondary mutations in the solvent-front region of the kinase (e.g. G810C/S/R) becomes a major challenge for selective RET inhibitor therapies. Herein, we report a structure-based design of 1-methyl-3-((4-(quinolin-4-yloxy)phenyl)amino)-1H-pyrazole-4-carboxamide derivatives as new RET kinase inhibitors which are capable of suppressing the RETG810 C/R resistant mutants. One of the representative compounds, 8q, potently suppressed wild-type RET kinase with an IC50 value of 13.7 nM. It also strongly inhibited the proliferation of BaF3 cells stably expressing various oncogenic fusions of RET kinase with solvent-front mutations, e.g. CCDC6-RETG810C, CCDC6-RETG810R, KIF5B-RETG810C and KIF5B-RETG810R, with IC50 values of 15.4, 53.2, 54.2 and 120.0 nM, respectively. Furthermore, 8q dose-dependently inhibited the activation of RET and downstream signals and obviously triggered apoptosis in Ba/F3-CCDC6-RETG810 C/R cells. The compound also exhibited significant anti-tumor efficacy with a tumor growth inhibition (TGI) value of 66.9% at 30 mg/kg/day via i. p. in a Ba/F3-CCDC6-RETG810C xenograft mouse model. Compound 8q may be utilized as a lead compound for drug discovery combating acquired resistance against selective RET inhibitor therapies.
中文翻译:

1-Methyl-3-((4-(quinolin-4-yloxy)phenyl)amino)-1H-pyrazole-4-carboxamide 衍生物在转染 (RET) 激酶抑制剂中重新排列,能够抑制溶剂前沿区域的抗性突变体
转染期间重排 (RET) 是抗癌药物发现的有效靶标,两种选择性 RET 抑制剂于 2020 年获得美国 FDA 批准。然而,由激酶溶剂前沿区域的二次突变介导的获得性耐药(例如G810C/S/ R) 成为选择性 RET 抑制剂治疗的主要挑战。在此,我们报告了 1-methyl-3-((4-(quinolin-4-yloxy)phenyl)amino)-1 H -pyrazole-4-carboxamide 衍生物的基于结构的设计作为新的 RET 激酶抑制剂,能够抑制 RET G810 C/R抗性突变体。代表性化合物之一8q可有效抑制野生型 RET 激酶,IC 50值为 13.7 nM。它还强烈抑制稳定表达 RET 激酶与溶剂前沿突变的各种致癌融合的 BaF3 细胞的增殖,例如CCDC6-RET G810C、CCDC6-RET G810R、KIF5B-RET G810C和 KIF5B-RET G810R,IC 50值为 15.4 , 53.2, 54.2 和 120.0 nM,分别。此外,8q剂量依赖性地抑制 RET 和下游信号的激活,并明显触发 Ba/F3-CCDC6-RET G810 C/R细胞凋亡。该化合物还表现出显着的抗肿瘤功效,在 30 mg/kg/天时,肿瘤生长抑制 (TGI) 值为 66.9%在 Ba/F3-CCDC6-RET G810C异种移植小鼠模型中进行 ip。化合物8q可用作药物发现的先导化合物,以对抗对选择性 RET 抑制剂疗法的获得性耐药性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号