Biochemical Engineering Journal ( IF 3.7 ) Pub Date : 2022-10-19 , DOI: 10.1016/j.bej.2022.108677 Runze Li , Xiaochen Liu , Xueping Li , Duoduo Tian , Daidi Fan , Xiaoxuan Ma , Zhansheng Wu
|
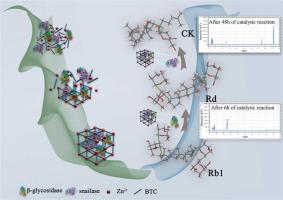
Enzyme immobilization via metal–organic frameworks is a key step to improve the stability and catalytic activity of the enzyme for realizing the enzymatic conversion of ginsenoside compound K (CK) in industrial application. Even though current studies mainly focused on improving the stability and reusability of enzymes through immobilization, the problem of long enzyme conversion cycle has not been solved. In this study, green synthesis of Zn-BTC co-immobilized snailase (SN) and β-glucosidase (β-G) was applied to design and construct β-G&SN@Zn-BTC (β-Glycosidase and snailase were co-immobilized on Zn-BTC) biocomposite. Results showed the β-G&SN@Zn-BTC materials have good stability and recyclability under extreme conditions (pH, temperature, and organic solvents). At pH 8, free β-G&SN was completely inactivated, whereas β-G&SN@Zn-BTC maintained 51.2% of the enzymatic activity. The conversion rate of CK with β-G&SN@Zn-BTC biocomposite was 1.73 times that of immobilization of single SN, indicating significant advantages over single enzyme immobilization in terms of transformation efficiency. This work provides a green, simple, low-cost, and sustainable strategy and lays the foundation for the industrial production of ginsenoside CK through innovative design.















































 京公网安备 11010802027423号
京公网安备 11010802027423号