当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Cyclopropanation with 4-Aryloxy-1-sulfonyl-1,2,3-triazoles: Expanding the Range of Rhodium-Stabilized Donor/Acceptor Carbenes to Systems with an Oxygen Donor Group
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-10-20 , DOI: 10.1021/acs.joc.2c00978 Robert W Kubiak 1 , William F Tracy 1 , Joshua S Alford 1 , Huw M L Davies 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-10-20 , DOI: 10.1021/acs.joc.2c00978 Robert W Kubiak 1 , William F Tracy 1 , Joshua S Alford 1 , Huw M L Davies 1
Affiliation
|
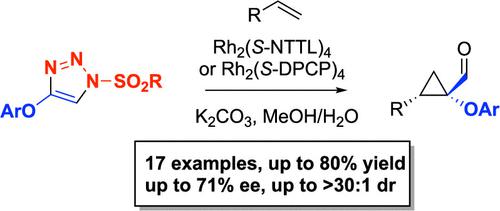
Rhodium-catalyzed enantioselective synthesis of 1-phenoxycyclopropane-1-carbaldehydes by intermolecular cyclopropanation of terminal alkenes followed by imine hydrolysis is described. This methodology utilizes 4-aryloxy-1-sulfonyl-1,2,3-triazoles as the carbene precursors and the chiral dirhodium(II) tetracarboxylates Rh2(S-NTTL)4 or Rh2(S-DPCP)4 as the catalysts. These reactions are considered to proceed via rhodium-stabilized donor/acceptor carbene intermediates, and these studies demonstrate that a heteroatom donor group is compatible with an enantioselective transformation.
中文翻译:

4-芳氧基-1-磺酰基-1,2,3-三唑的不对称环丙烷化:将铑稳定的供体/受体卡宾的范围扩展到具有氧供体基团的系统
描述了通过末端烯烃的分子间环丙烷化随后亚胺水解,铑催化对映选择性合成 1-苯氧基环丙烷-1-甲醛。该方法利用 4-芳氧基-1-磺酰基-1,2,3-三唑作为卡宾前体,使用手性四羧酸二铑(II) Rh 2 ( S -NTTL) 4或 Rh 2 ( S -DPCP) 4作为催化剂。这些反应被认为是通过铑稳定的供体/受体卡宾中间体进行的,并且这些研究表明杂原子供体基团与对映选择性转化相容。
更新日期:2022-10-20
中文翻译:

4-芳氧基-1-磺酰基-1,2,3-三唑的不对称环丙烷化:将铑稳定的供体/受体卡宾的范围扩展到具有氧供体基团的系统
描述了通过末端烯烃的分子间环丙烷化随后亚胺水解,铑催化对映选择性合成 1-苯氧基环丙烷-1-甲醛。该方法利用 4-芳氧基-1-磺酰基-1,2,3-三唑作为卡宾前体,使用手性四羧酸二铑(II) Rh 2 ( S -NTTL) 4或 Rh 2 ( S -DPCP) 4作为催化剂。这些反应被认为是通过铑稳定的供体/受体卡宾中间体进行的,并且这些研究表明杂原子供体基团与对映选择性转化相容。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号