当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Super-Resolution Imaging of Autophagy by a Preferred Pair of Self-Labeling Protein Tags and Fluorescent Ligands
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-10-19 , DOI: 10.1021/acs.analchem.2c03125 Huizi Man 1 , Lin Zhou 1 , Guanghao Zhu 2 , Ying Zheng 1 , Zhiwei Ye 1 , Zhenlong Huang 1 , Xinru Teng 3 , Chunzhi Ai 3 , Guangbo Ge 2 , Yi Xiao 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-10-19 , DOI: 10.1021/acs.analchem.2c03125 Huizi Man 1 , Lin Zhou 1 , Guanghao Zhu 2 , Ying Zheng 1 , Zhiwei Ye 1 , Zhenlong Huang 1 , Xinru Teng 3 , Chunzhi Ai 3 , Guangbo Ge 2 , Yi Xiao 1
Affiliation
|
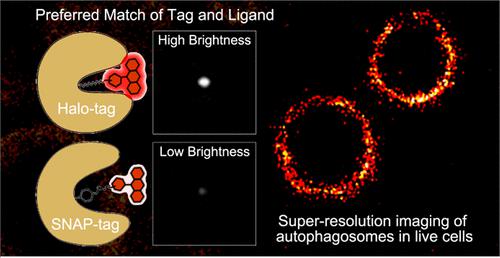
Autophagy is a core recycling process for homeostasis, with its dysfunction associated with tumorigenesis and various diseases. Yet, its subtle intracellular details are covered due to the limited resolution of conventional microscopies. The major challenge for modern super-resolution microscopy deployment is the lack of a practical labeling system, which could provide robust fluorescence with fidelity in the context of the dynamic autophagy microenvironment. Herein, a representative autophagy marker LC3 protein is selected to develop two hybrid self-labeling systems with tetramethylrhodamine (TMR) fluorophores through SNAP/Halo-tag technologies. A systematic investigation indicated that the match of the LC3-Halo and TMR ligand remarkably outperforms that of LC3-SNAP, as the former Halo system exhibited more robust single-molecule brightness (440 vs 247), total photon numbers (45600 vs 13500), and dwell time of the initial bright state (0.82 vs 0.40 s) than the latter. With the aid of this desirable Halo system, for the first time, live-cell ferritinophagy is monitored with a spatial resolution of ∼50 nm, which disclosed reduced sizes of autophagosomes (∼650 nm, ferritinophagy) than those in nonselective (∼840 nm, mammalian target of rapamycin (mTOR)) and selective autophagy (∼900 nm, mitophagy).
中文翻译:

通过一对首选的自标记蛋白标签和荧光配体对自噬进行超分辨率成像
自噬是体内平衡的核心循环过程,其功能障碍与肿瘤发生和各种疾病有关。然而,由于传统显微镜的分辨率有限,其细微的细胞内细节被掩盖了。现代超分辨率显微镜部署的主要挑战是缺乏实用的标记系统,该系统可以在动态自噬微环境的背景下提供具有保真度的稳健荧光。在此,选择具有代表性的自噬标志物LC3蛋白,通过SNAP/Halo-tag技术开发两种具有四甲基罗丹明(TMR)荧光团的混合自标记系统。系统研究表明,LC3-Halo 和 TMR 配体的匹配显着优于 LC3-SNAP,因为前者的 Halo 系统表现出比后者更强大的单分子亮度(440 对 247)、总光子数(45600 对 13500)和初始亮态的停留时间(0.82 对 0.40 s)。借助这种理想的 Halo 系统,首次以 ~50 nm 的空间分辨率监测活细胞铁蛋白吞噬,这表明自噬体(~650 nm,铁蛋白噬)的尺寸比非选择性(~840 nm)小,哺乳动物雷帕霉素靶标(mTOR))和选择性自噬(~900 nm,线粒体自噬)。
更新日期:2022-10-19
中文翻译:

通过一对首选的自标记蛋白标签和荧光配体对自噬进行超分辨率成像
自噬是体内平衡的核心循环过程,其功能障碍与肿瘤发生和各种疾病有关。然而,由于传统显微镜的分辨率有限,其细微的细胞内细节被掩盖了。现代超分辨率显微镜部署的主要挑战是缺乏实用的标记系统,该系统可以在动态自噬微环境的背景下提供具有保真度的稳健荧光。在此,选择具有代表性的自噬标志物LC3蛋白,通过SNAP/Halo-tag技术开发两种具有四甲基罗丹明(TMR)荧光团的混合自标记系统。系统研究表明,LC3-Halo 和 TMR 配体的匹配显着优于 LC3-SNAP,因为前者的 Halo 系统表现出比后者更强大的单分子亮度(440 对 247)、总光子数(45600 对 13500)和初始亮态的停留时间(0.82 对 0.40 s)。借助这种理想的 Halo 系统,首次以 ~50 nm 的空间分辨率监测活细胞铁蛋白吞噬,这表明自噬体(~650 nm,铁蛋白噬)的尺寸比非选择性(~840 nm)小,哺乳动物雷帕霉素靶标(mTOR))和选择性自噬(~900 nm,线粒体自噬)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号