当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sterically Hindered Diarylethenes with a Benzobis(thiadiazole) Bridge: Enantiospecific Transformation and Reversible Photosuperstructures
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-10-19 , DOI: 10.1021/acs.accounts.2c00419 Mengqi Li 1 , Wei-Hong Zhu 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-10-19 , DOI: 10.1021/acs.accounts.2c00419 Mengqi Li 1 , Wei-Hong Zhu 1
Affiliation
|
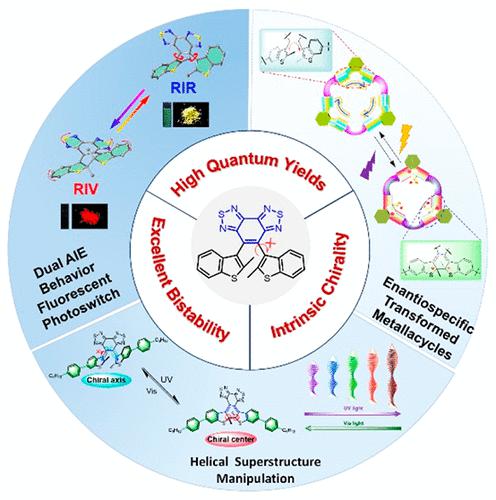
Photochromic diarylethenes featuring reversible regulation by external light irradiation have attracted increasing attention in versatile applications such as logic gates, supramolecular systems, liquid crystals, and super-resolution imaging because of their outstanding bistability and fatigue resistance. However, for typical diarylethene systems, there always exist three typical unsolved issues. The first is how to modulate the bistability between the open and closed forms from the viewpoint of ethene bridge aromaticity. The second is how to decrease and avoid the photoinactive parallel conformer in order to achieve a high quantum yield, since the open form possesses the photoactive antiparallel (ap) conformation and the photoinactive parallel (p) conformation. Because of the typical rapid rotation of the flexible side aryl groups, the two conformers cannot be separated efficiently, thereby resulting in a relatively low photocyclization quantum yield. The third is how to fulfill the enantiospecific transformation with reversibility to photomodulate the chirality. Stereochemically, the ap conformer with C2 symmetry can be further subdivided into a pair of enantiomers with P and M helicity originating from the central hexatriene moiety. Similarly, the rapid rotation can also lead to the loss of intrinsic chirality, restricting the development and application of light-driven chiroptical switches. Accordingly, it is desirable to construct a specific diarylethene system to break through these bottlenecks for real versatile applications.
中文翻译:

具有苯并双(噻二唑)桥的空间位阻二芳基乙烯:对映体特异性转化和可逆光超结构
光致变色二芳基乙烯具有通过外部光照射进行可逆调节的特性,由于其出色的双稳态和抗疲劳性,在逻辑门、超分子系统、液晶和超分辨率成像等多种应用中引起了越来越多的关注。然而,对于典型的二芳基乙烯体系,始终存在三个典型的未解决问题。首先是如何从乙烯桥芳香性的角度调节开放和封闭形式之间的双稳态。第二个是如何减少和避免光非活性平行构象以实现高量子产率,因为开放形式具有光活性反平行(ap)构象和光非活性平行(p) 构象。由于柔性侧芳基的典型快速旋转,两个构象异构体不能有效分离,从而导致相对较低的光环化量子产率。三是如何通过可逆性实现对映体特异性转化来光调节手性。立体化学上,具有C 2对称性的ap构象异构体可以进一步细分为一对具有P和M的对映异构体源自中心己三烯部分的螺旋性。同样,快速旋转也会导致本征手性的丧失,限制了光驱动手性光开关的开发和应用。因此,需要构建一种特定的二芳基乙烯体系,以突破这些瓶颈,实现真正的多功能应用。
更新日期:2022-10-19
中文翻译:

具有苯并双(噻二唑)桥的空间位阻二芳基乙烯:对映体特异性转化和可逆光超结构
光致变色二芳基乙烯具有通过外部光照射进行可逆调节的特性,由于其出色的双稳态和抗疲劳性,在逻辑门、超分子系统、液晶和超分辨率成像等多种应用中引起了越来越多的关注。然而,对于典型的二芳基乙烯体系,始终存在三个典型的未解决问题。首先是如何从乙烯桥芳香性的角度调节开放和封闭形式之间的双稳态。第二个是如何减少和避免光非活性平行构象以实现高量子产率,因为开放形式具有光活性反平行(ap)构象和光非活性平行(p) 构象。由于柔性侧芳基的典型快速旋转,两个构象异构体不能有效分离,从而导致相对较低的光环化量子产率。三是如何通过可逆性实现对映体特异性转化来光调节手性。立体化学上,具有C 2对称性的ap构象异构体可以进一步细分为一对具有P和M的对映异构体源自中心己三烯部分的螺旋性。同样,快速旋转也会导致本征手性的丧失,限制了光驱动手性光开关的开发和应用。因此,需要构建一种特定的二芳基乙烯体系,以突破这些瓶颈,实现真正的多功能应用。





























 京公网安备 11010802027423号
京公网安备 11010802027423号