当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoinduced Promiscuity of Cyclohexanone Monooxygenase for the Enantioselective Synthesis of α-Fluoroketones
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-10-19 , DOI: 10.1002/anie.202211199 Yongzhen Peng 1 , Zhiguo Wang 2 , Yang Chen 1 , Weihua Xu 1 , Yujing Hu 1 , Zhichun Chen 1 , Jian Xu 3 , Qi Wu 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-10-19 , DOI: 10.1002/anie.202211199 Yongzhen Peng 1 , Zhiguo Wang 2 , Yang Chen 1 , Weihua Xu 1 , Yujing Hu 1 , Zhichun Chen 1 , Jian Xu 3 , Qi Wu 1
Affiliation
|
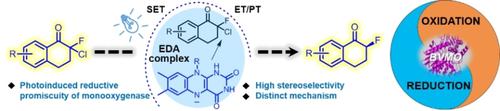
The photoinduced reductive dehalogenation promiscuity of cyclohexanone monooxygenase (CHMO) with a novel mechanism of ET/PT distinct from the photoinduced promiscuity of natural reductases is reported. Various highly enantioenriched α-fluoroketones were synthesized by photoinduced reductive dehalogenation of α,α-halofluoroketones in a process catalyzed by the rationally designed CHMO mutants.
中文翻译:

光诱导环己酮单加氧酶的混杂对映选择性合成 α-氟代酮
报道了环己酮单加氧酶 (CHMO) 的光诱导还原脱卤混杂与 ET/PT 的新机制不同于天然还原酶的光诱导混杂。在合理设计的 CHMO 突变体催化的过程中,通过 α,α-卤代氟酮的光诱导还原脱卤合成了各种高度对映体富集的 α-氟酮。
更新日期:2022-10-19
中文翻译:

光诱导环己酮单加氧酶的混杂对映选择性合成 α-氟代酮
报道了环己酮单加氧酶 (CHMO) 的光诱导还原脱卤混杂与 ET/PT 的新机制不同于天然还原酶的光诱导混杂。在合理设计的 CHMO 突变体催化的过程中,通过 α,α-卤代氟酮的光诱导还原脱卤合成了各种高度对映体富集的 α-氟酮。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号