当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toward Understanding the Effect of Fluoride Ions on the Solvation Structure in Lithium Metal Batteries: Insights from First-Principles Simulations
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-10-19 , DOI: 10.1021/acsami.2c14770 Jianhui Zheng 1 , Yao Wang 1 , Juncheng Wang 1 , Huadong Yuan 1 , Yujing Liu 1 , Tiefeng Liu 1 , Jianmin Luo 1 , Jianwei Nai 1 , Xinyong Tao 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-10-19 , DOI: 10.1021/acsami.2c14770 Jianhui Zheng 1 , Yao Wang 1 , Juncheng Wang 1 , Huadong Yuan 1 , Yujing Liu 1 , Tiefeng Liu 1 , Jianmin Luo 1 , Jianwei Nai 1 , Xinyong Tao 1
Affiliation
|
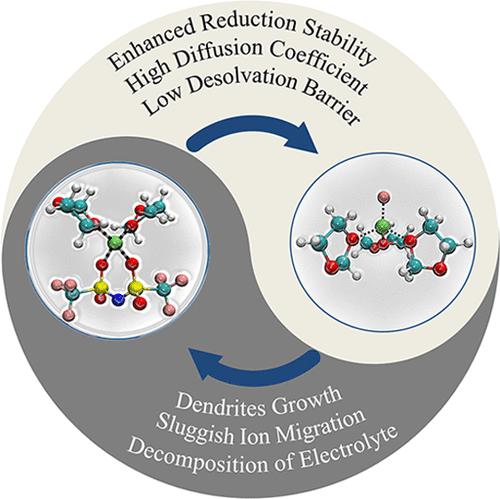
Regulating the structure and composition of the lithium-ion (Li+) solvation shell is crucial to the performance of lithium metal batteries. The introduction of fluorine anions (F–) into the electrolyte significantly enhances the cycle efficiency and the interfacial stability of lithium metal anodes. However, the effect of dissolved F– on the solvation shell is rarely touched in the literature. Herein, we investigate the evolution processing of the fluorine-containing solvation structure to explore the underlying mechanisms via first-principles calculations. The additive F– is found to invade the first solvation shell and strongly coordinate with Li+, liberating the bis(trifluoromethanesulfonyl) imide anion (TFSI–) from the Li+ local environment, which enhances the Li+ diffusivity by altering the transport mode. Moreover, the fluorine-containing Li+ solvation shell exhibits a higher lowest unoccupied molecular orbital energy level than that of the solvation sheath without F– additives, suggesting the reduction stability of the electrolyte. Furthermore, the Gibbs free energy calculations for Li+ desolvation reveal that the energy barrier of the Li+ desolvation process will be reduced because of the presence of F–. Our work provides new insights into the mechanisms of electrolyte fluorinated strategies and leads to the rational design of high-performance lithium metal batteries.
中文翻译:

了解氟离子对锂金属电池溶剂化结构的影响:第一性原理模拟的见解
调节锂离子 (Li + ) 溶剂化壳的结构和组成对锂金属电池的性能至关重要。在电解质中引入氟阴离子(F- )显着提高了锂金属负极的循环效率和界面稳定性。然而,文献中很少提及溶解的 F -对溶剂化壳的影响。在此,我们研究了含氟溶剂化结构的演化过程,以通过第一性原理计算探索其潜在机制。发现添加剂 F -侵入第一个溶剂化壳层并与 Li +强配位,从Li +局部环境中释放双(三氟甲磺酰基)酰亚胺阴离子(TFSI - ),通过改变传输模式来增强Li +扩散率。此外,含氟Li +溶剂化壳层的最低未占分子轨道能级比没有F -添加剂的溶剂化壳层更高,表明电解质的还原稳定性。此外,Li +去溶剂化的 Gibbs 自由能计算表明,由于F -. 我们的工作为电解质氟化策略的机制提供了新的见解,并导致了高性能锂金属电池的合理设计。
更新日期:2022-10-19
中文翻译:

了解氟离子对锂金属电池溶剂化结构的影响:第一性原理模拟的见解
调节锂离子 (Li + ) 溶剂化壳的结构和组成对锂金属电池的性能至关重要。在电解质中引入氟阴离子(F- )显着提高了锂金属负极的循环效率和界面稳定性。然而,文献中很少提及溶解的 F -对溶剂化壳的影响。在此,我们研究了含氟溶剂化结构的演化过程,以通过第一性原理计算探索其潜在机制。发现添加剂 F -侵入第一个溶剂化壳层并与 Li +强配位,从Li +局部环境中释放双(三氟甲磺酰基)酰亚胺阴离子(TFSI - ),通过改变传输模式来增强Li +扩散率。此外,含氟Li +溶剂化壳层的最低未占分子轨道能级比没有F -添加剂的溶剂化壳层更高,表明电解质的还原稳定性。此外,Li +去溶剂化的 Gibbs 自由能计算表明,由于F -. 我们的工作为电解质氟化策略的机制提供了新的见解,并导致了高性能锂金属电池的合理设计。






























 京公网安备 11010802027423号
京公网安备 11010802027423号