Chemical Engineering Science ( IF 4.1 ) Pub Date : 2022-10-17 , DOI: 10.1016/j.ces.2022.118216
Fotis P. Rigas
|
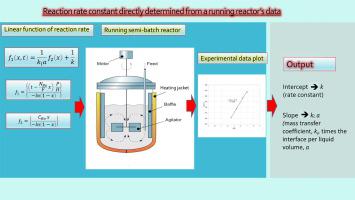
This study aims at determining both the rate constants of chlorination of p-xylene with molecular chlorine and FeCl3 as a catalyst at 70 °C and 90 °C and the related chemical engineering parameters to be used in the design of an effective chemical reactor.
This was accomplished by a novel approach of constructing a linear mathematical function for a gas–liquid semi-batch agitated reactor correlating all engaged physical and chemical factors. Collecting values for all the necessary parameters for the application of the function, or in the absence of them calculating them, and exploiting the intercept and slope of the linear function became possible the apparent and the catalytic reaction rate constants to be calculated. It was found that the calculated values were of the same order of magnitude as values obtained by other researchers.
The parameters needed to be calculated were Henry’s constant, the diffusion coefficient of the solute A (chlorine) to the solvent B (p = xylene), DAB, and the mean liquid phase mass transfer coefficient, kl. Other parameters of chemical engineering interest were also determined, namely, the interface per unit volume of liquid, a, the thickness of the liquid film, δl, and the Hatta number, MH.
Evaluating the Hatta number estimated, it was concluded that the chlorination of p-xylene with molecular chlorine is very slow at both temperatures investigated, and almost no reaction occurs in the film compared to the bulk of the liquid. Thus, to increase the overall reaction rate, a large volume of liquid is needed while the increase of agitation intensity is of no value here.
Combining the rate constant ratios obtained recently for the four consecutive second-order irreversible chlorinations of p-xylene with molecular chlorine with FeCl3 as a catalyst at 70 °C and under identical conditions it was possible to determine the values of all rate constants up to the fourth chlorinated product.
中文翻译:

气液搅拌反应器中对二甲苯氯化的化学工程动力学
本研究旨在确定在 70 °C 和 90 °C 下以分子氯和 FeCl 3作为催化剂对二甲苯的氯化速率常数以及用于设计有效化学反应器的相关化学工程参数。
这是通过一种新颖的方法来实现的,该方法为气液半间歇搅拌反应器构建线性数学函数,将所有参与的物理和化学因素关联起来。为应用函数收集所有必要参数的值,或者在没有它们的情况下计算它们,并利用线性函数的截距和斜率,可以计算表观和催化反应速率常数。结果发现,计算值与其他研究人员获得的值处于同一数量级。
需要计算的参数是亨利常数、溶质A(氯)到溶剂B(p = 二甲苯)的扩散系数D AB和平均液相传质系数k l。还确定了化学工程感兴趣的其他参数,即每单位体积液体的界面a、液膜厚度δ l和哈塔数M H。
评估估计的 Hatta 数,得出的结论是,在所研究的两个温度下,对二甲苯与分子氯的氯化反应都非常缓慢,并且与大部分液体相比,薄膜中几乎没有发生反应。因此,为了提高整体反应速率,需要大量的液体,而增加搅拌强度在这里没有任何价值。
结合最近获得的对二甲苯与分子氯在 70 °C 下以 FeCl 3作为催化剂的四次连续二级不可逆氯化的速率常数比,在相同条件下,可以确定所有速率常数的值高达第四种氯化产物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号