当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alternating Isotactic Polyhydroxyalkanoates via Site- and Stereoselective Polymerization of Unsymmetrical Diolides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-10-18 , DOI: 10.1021/jacs.2c08791 Zhen Zhang 1 , Changxia Shi 1 , Miriam Scoti 1, 2 , Xiaoyan Tang 1 , Eugene Y-X Chen 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-10-18 , DOI: 10.1021/jacs.2c08791 Zhen Zhang 1 , Changxia Shi 1 , Miriam Scoti 1, 2 , Xiaoyan Tang 1 , Eugene Y-X Chen 1
Affiliation
|
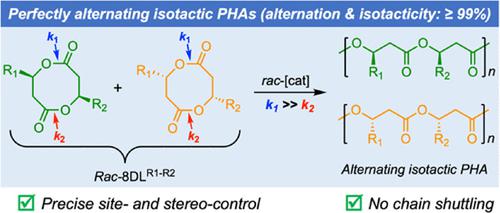
Naturally produced, biodegradable polyhydroxyalkanoates (PHAs) promise more sustainable alternatives to nonrenewable/degradable plastics, but biological PHA’s stereomicrostructures are strictly confined to isotactic (R)-polymers or copolymers of random sequences. Chemical synthesis via catalyzed ring-opening polymerization (ROP) of cyclic (di)esters offers expedient access to diverse PHA microstructures, including those with defined comonomer sequences and tacticities. However, the synthesis of alternating isotactic PHAs has not been achieved by the existing methodologies. Here, we report the design of unsymmetrically disubstituted eight-membered diolides (rac-8DLR1-R2) and their site- and stereoselective ROP with discrete chiral catalysts, enabling the synthesis of alternating isotactic PHAs, poly(3-hydroxybutyrate-alt-3-hydroxyvalerate) (alt-P3HBV) and poly(3-hydroxybutyrate-alt-3-hydroxyheptanoate) (alt-P3HBHp), with high to quantitative (>99%) alternation and isotacticity and Mn up to 113 kDa and Đ = 1.01. Physical properties of such PHAs are substantially determined by the degree of backbone sequence alternation and tacticity, ranging from amorphous to semi-crystalline materials. The alt-P3HBV shows significantly improved mechanical performance relative to the constituent homopolymers. Intriguingly, enantiomeric (R)-alt-P3HBV and (S)-alt-P3HBV, synthesized by kinetically resolved ROP of rac-8DLMe-Et, form a stereocomplex with a significantly enhanced Tm (by 53 °C), while the enantiomeric homopolymers do not form a stereocomplex.
中文翻译:

通过不对称二醇的位点和立体选择性聚合交替等规聚羟基链烷酸酯
天然产生的可生物降解聚羟基脂肪酸酯 (PHA) 有望成为不可再生/可降解塑料的更可持续替代品,但生物 PHA 的立体微观结构严格限于等规 ( R )- 聚合物或随机序列的共聚物。通过环状(二)酯的催化开环聚合 (ROP) 进行化学合成可以方便地获得各种 PHA 微观结构,包括那些具有确定的共聚单体序列和立构规整度的微观结构。然而,现有的方法尚未实现交替全同 PHA 的合成。在这里,我们报告了不对称双取代的八元二酯的设计 ( rac -8DL R1-R2) 及其具有离散手性催化剂的位点和立体选择性 ROP,能够合成交替全同立构 PHA、聚(3-羟基丁酸酯-alt-3-羟基戊酸酯)(alt - P3HBV)和聚(3-羟基丁酸酯-alt-3-羟基庚酸酯) ) ( alt -P3HBHp),具有高到定量 (>99%) 的交替和全同立构规整度,M n高达 113 kDa 和Đ = 1.01。这种 PHA 的物理性质基本上由主链序列交替和立构规整度的程度决定,范围从无定形到半结晶材料。另类_-P3HBV 相对于组成均聚物显示出显着改善的机械性能。有趣的是,对映体 ( R )-alt- P3HBV和 ( S )-alt- P3HBV ,由rac -8DL Me-Et的动力学解析 ROP 合成,形成具有显着增强的Tm(53°C)的立体复合物,而对映体均聚物不形成立构复合物。
更新日期:2022-10-18
中文翻译:

通过不对称二醇的位点和立体选择性聚合交替等规聚羟基链烷酸酯
天然产生的可生物降解聚羟基脂肪酸酯 (PHA) 有望成为不可再生/可降解塑料的更可持续替代品,但生物 PHA 的立体微观结构严格限于等规 ( R )- 聚合物或随机序列的共聚物。通过环状(二)酯的催化开环聚合 (ROP) 进行化学合成可以方便地获得各种 PHA 微观结构,包括那些具有确定的共聚单体序列和立构规整度的微观结构。然而,现有的方法尚未实现交替全同 PHA 的合成。在这里,我们报告了不对称双取代的八元二酯的设计 ( rac -8DL R1-R2) 及其具有离散手性催化剂的位点和立体选择性 ROP,能够合成交替全同立构 PHA、聚(3-羟基丁酸酯-alt-3-羟基戊酸酯)(alt - P3HBV)和聚(3-羟基丁酸酯-alt-3-羟基庚酸酯) ) ( alt -P3HBHp),具有高到定量 (>99%) 的交替和全同立构规整度,M n高达 113 kDa 和Đ = 1.01。这种 PHA 的物理性质基本上由主链序列交替和立构规整度的程度决定,范围从无定形到半结晶材料。另类_-P3HBV 相对于组成均聚物显示出显着改善的机械性能。有趣的是,对映体 ( R )-alt- P3HBV和 ( S )-alt- P3HBV ,由rac -8DL Me-Et的动力学解析 ROP 合成,形成具有显着增强的Tm(53°C)的立体复合物,而对映体均聚物不形成立构复合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号