Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Condensation of acrylonitrile and aryl acetonitrile: construction of α-amino-β-cyano cyclohexene skeletons
RSC Advances ( IF 3.9 ) Pub Date : 2022-10-18 , DOI: 10.1039/d2ra04936h Wei Zhang 1 , Chuan-Su Tang 2 , Shi-Qun Xiang 3, 4
RSC Advances ( IF 3.9 ) Pub Date : 2022-10-18 , DOI: 10.1039/d2ra04936h Wei Zhang 1 , Chuan-Su Tang 2 , Shi-Qun Xiang 3, 4
Affiliation
|
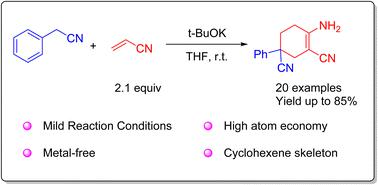
A representative condensation of acrylonitrile and aryl acetonitrile has been reported for the synthesis of α-amino-β-cyano cyclohexene. The reaction was carried out mildly in an open environment at room temperature. The scope and versatility of the method have been demonstrated with 20 examples, containing highly active ethynyl groups. Further applications for 4-aminopyrimidine compounds were performed. A mechanism was proposed, involving Michael additions between acrylonitrile and aryl acetonitriles as well as intramolecular condensation.
中文翻译:

丙烯腈和芳基乙腈的缩合:α-氨基-β-氰基环己烯骨架的构建
据报道,丙烯腈和芳基乙腈的代表性缩合反应可用于合成 α-氨基-β-氰基环己烯。反应在室温下在开放环境中温和地进行。该方法的范围和多功能性已通过 20 个包含高活性乙炔基的示例得到证明。对 4-氨基嘧啶化合物进行了进一步的应用。提出了一种机理,涉及丙烯腈和芳基乙腈之间的迈克尔加成以及分子内缩合。
更新日期:2022-10-18
中文翻译:

丙烯腈和芳基乙腈的缩合:α-氨基-β-氰基环己烯骨架的构建
据报道,丙烯腈和芳基乙腈的代表性缩合反应可用于合成 α-氨基-β-氰基环己烯。反应在室温下在开放环境中温和地进行。该方法的范围和多功能性已通过 20 个包含高活性乙炔基的示例得到证明。对 4-氨基嘧啶化合物进行了进一步的应用。提出了一种机理,涉及丙烯腈和芳基乙腈之间的迈克尔加成以及分子内缩合。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号