当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silyl-mediated photoredox-catalyzed radical–radical cross-coupling reaction of alkyl bromides and ketoesters
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-10-18 , DOI: 10.1039/d2qo01377k
Hao-Luo Jiang 1 , Yu-Hao Yang 1 , Ya-Nan Zhao 2 , Yan-Hong He 1 , Zhi Guan 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-10-18 , DOI: 10.1039/d2qo01377k
Hao-Luo Jiang 1 , Yu-Hao Yang 1 , Ya-Nan Zhao 2 , Yan-Hong He 1 , Zhi Guan 1
Affiliation
|
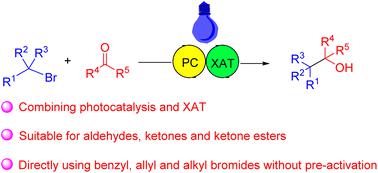
Direct reductive cleavage of unactivated alkyl halides to radical intermediates is challenging, whereas activation of alkyl halides via halogen atom transfer by silyl radicals is facile. Here, a strategy for cross-coupling between organic bromides and carbonyl compounds is developed by combining photocatalysis and halogen atom transfer using a photocatalyst and tris(trimethylsilyl)silane. In this way, aldehydes, ketones and ketone esters can be coupled with benzyl bromides, allyl bromides and alkyl bromides respectively, to give the corresponding alcohols under mild conditions.
中文翻译:

硅烷介导的光氧化还原催化的烷基溴和酮酯的自由基交叉偶联反应
将未活化的卤代烷直接还原裂解为自由基中间体具有挑战性,而通过甲硅烷基自由基的卤素原子转移来活化卤代烷是容易的。在这里,通过使用光催化剂和三(三甲基甲硅烷基)硅烷将光催化和卤素原子转移相结合,开发了一种有机溴化物和羰基化合物之间的交叉偶联策略。这样,醛、酮和酮酯可以分别与苄基溴、烯丙基溴和烷基溴偶联,在温和条件下得到相应的醇。
更新日期:2022-10-21
中文翻译:

硅烷介导的光氧化还原催化的烷基溴和酮酯的自由基交叉偶联反应
将未活化的卤代烷直接还原裂解为自由基中间体具有挑战性,而通过甲硅烷基自由基的卤素原子转移来活化卤代烷是容易的。在这里,通过使用光催化剂和三(三甲基甲硅烷基)硅烷将光催化和卤素原子转移相结合,开发了一种有机溴化物和羰基化合物之间的交叉偶联策略。这样,醛、酮和酮酯可以分别与苄基溴、烯丙基溴和烷基溴偶联,在温和条件下得到相应的醇。































 京公网安备 11010802027423号
京公网安备 11010802027423号