当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Constructing Air-Stable and Reconstruction-Inhibited Transition Metal Sulfide Catalysts via Tailoring Electron-Deficient Distribution for Water Oxidation
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-10-17 , DOI: 10.1021/acscatal.2c03338 Runzhe Chen 1 , Zeyi Zhang 1 , Zichen Wang 1 , Wei Wu 1 , Shaowu Du 2 , Wangbin Zhu 1 , Haifeng Lv 3 , Niancai Cheng 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-10-17 , DOI: 10.1021/acscatal.2c03338 Runzhe Chen 1 , Zeyi Zhang 1 , Zichen Wang 1 , Wei Wu 1 , Shaowu Du 2 , Wangbin Zhu 1 , Haifeng Lv 3 , Niancai Cheng 1
Affiliation
|
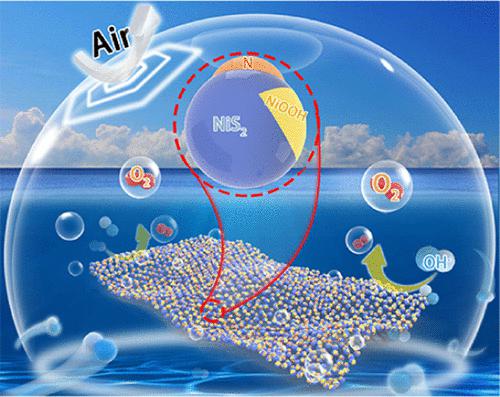
In promising transition metal sulfide catalysts, the extraordinary instability under air exposure and oxygen evolution reaction (OER) catalysis severely degrades their activity and stability in the electrochemical water splitting reaction, inhibiting their practical applications. Herein, guided by a theoretical mechanism study, it is disclosed that the adsorbing ability and electronic interaction for molecular oxygen will be significantly weakened in nickel disulfide (NiS2) by constructing an electron-deficient distribution on Ni–S sites with N atom introduction, which efficiently inhibits the process of O2 adsorption and electrophilic activation during oxidation, thus achieving air-stable capacity for NiS2. In addition, theoretical calculations further reveal that such an electronic redistribution will weaken the OH– adsorption on NiS2 and thus inhibit the reconstruction process during the OER process. Inspired by this, NiS2 nanosheets (NiS2 NSs) are synthesized and N atoms are introduced to bridge with Ni and S, resulting in electron-deficient Ni and S sites in N atom-bridged NiS2 NSs (N–NiS2 NSs). As expected, only 28.1% of the NiS2 phase is oxidized into sulfate nickel in N–NiS2 NSs after one month of air exposure with only 13 mV overpotential degradation toward the OER, while for NiS2 NSs, a fast and drastic phase transformation is undergone, resulting in 155 mV OER decline. For the OER process, the reconstruction from sulfides to (oxy)hydroxides is deservedly inhibited in such N–NiS2 NSs, with an in situ constructed N–NiS2/NiOOH heterostructure as an OER active phase, which exhibits higher OER activity and stability compared to those of completely NiOOH-oxidized NiS2 NSs. Rationalized by density functional theory (DFT) calculations, the N–NiS2/NiOOH heterostructure features a strong electron rearrangement at the interface, thus improving the chemisorption ability and conductivity compared to those of pristine NiOOH. Moreover, such a strategy of improving the air stability is also valid for other transition metal sulfides (TMS) (such as CoS2 and FeS2).
中文翻译:

通过调整水氧化的缺电子分布构建空气稳定和抑制重构的过渡金属硫化物催化剂
在有前景的过渡金属硫化物催化剂中,空气暴露和析氧反应(OER)催化下的异常不稳定性严重降低了它们在电化学水分解反应中的活性和稳定性,抑制了它们的实际应用。在此,在理论机理研究的指导下,揭示了通过引入 N 原子在 Ni-S 位点上构建缺电子分布,二硫化镍 (NiS 2 )中分子氧的吸附能力和电子相互作用将显着减弱,有效抑制氧化过程中的O 2吸附和亲电活化过程,从而实现对NiS 2的空气稳定容量. 此外,理论计算进一步表明,这种电子再分布会削弱NiS 2上的 OH 吸附,从而抑制 OER 过程中的重建过程。受此启发,合成了 NiS 2纳米片 (NiS 2 NSs),并引入 N 原子与 Ni 和 S 桥接,从而在 N 原子桥接的 NiS 2 NSs (N-NiS 2 NSs)中产生缺电子的 Ni 和 S 位点. 正如预期的那样,在空气暴露一个月后,在 N-NiS 2 NSs 中,只有 28.1% 的 NiS 2相被氧化成硫酸镍,向 OER 的过电位仅降解 13 mV,而对于 NiS 2NSs,经历了快速而剧烈的相变,导致 155 mV OER 下降。对于 OER 过程,在这种 N-NiS 2 NSs 中,从硫化物到(氧)氢氧化物的重构受到了应有的抑制,原位构建的 N-NiS 2 /NiOOH 异质结构作为 OER 活性相,具有更高的 OER 活性和稳定性与完全 NiOOH 氧化的 NiS 2 NSs 相比。通过密度泛函理论 (DFT) 计算合理化,N-NiS 2/NiOOH异质结构在界面处具有很强的电子重排,因此与原始NiOOH相比,其化学吸附能力和电导率有所提高。此外,这种提高空气稳定性的策略也适用于其他过渡金属硫化物(TMS)(如CoS 2和FeS 2)。
更新日期:2022-10-17
中文翻译:

通过调整水氧化的缺电子分布构建空气稳定和抑制重构的过渡金属硫化物催化剂
在有前景的过渡金属硫化物催化剂中,空气暴露和析氧反应(OER)催化下的异常不稳定性严重降低了它们在电化学水分解反应中的活性和稳定性,抑制了它们的实际应用。在此,在理论机理研究的指导下,揭示了通过引入 N 原子在 Ni-S 位点上构建缺电子分布,二硫化镍 (NiS 2 )中分子氧的吸附能力和电子相互作用将显着减弱,有效抑制氧化过程中的O 2吸附和亲电活化过程,从而实现对NiS 2的空气稳定容量. 此外,理论计算进一步表明,这种电子再分布会削弱NiS 2上的 OH 吸附,从而抑制 OER 过程中的重建过程。受此启发,合成了 NiS 2纳米片 (NiS 2 NSs),并引入 N 原子与 Ni 和 S 桥接,从而在 N 原子桥接的 NiS 2 NSs (N-NiS 2 NSs)中产生缺电子的 Ni 和 S 位点. 正如预期的那样,在空气暴露一个月后,在 N-NiS 2 NSs 中,只有 28.1% 的 NiS 2相被氧化成硫酸镍,向 OER 的过电位仅降解 13 mV,而对于 NiS 2NSs,经历了快速而剧烈的相变,导致 155 mV OER 下降。对于 OER 过程,在这种 N-NiS 2 NSs 中,从硫化物到(氧)氢氧化物的重构受到了应有的抑制,原位构建的 N-NiS 2 /NiOOH 异质结构作为 OER 活性相,具有更高的 OER 活性和稳定性与完全 NiOOH 氧化的 NiS 2 NSs 相比。通过密度泛函理论 (DFT) 计算合理化,N-NiS 2/NiOOH异质结构在界面处具有很强的电子重排,因此与原始NiOOH相比,其化学吸附能力和电导率有所提高。此外,这种提高空气稳定性的策略也适用于其他过渡金属硫化物(TMS)(如CoS 2和FeS 2)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号