当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold(I)-Catalyzed Diastereo- and Enantioselective 1,3-Dipolar Cycloaddition and Mannich Reactions of Azlactones
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-03-16 , DOI: 10.1021/ja1095045
Asa D Melhado 1 , Giovanni W Amarante , Z Jane Wang , Marco Luparia , F Dean Toste
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-03-16 , DOI: 10.1021/ja1095045
Asa D Melhado 1 , Giovanni W Amarante , Z Jane Wang , Marco Luparia , F Dean Toste
Affiliation
|
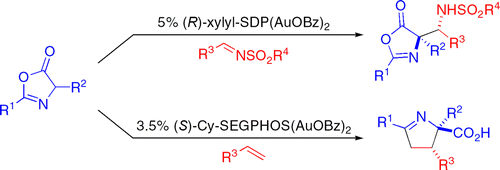
Azlactones participate in stereoselective reactions with electron-deficient alkenes and N-sulfonyl aldimines to give products of 1,3-dipolar cycloaddition and Mannich addition reactions, respectively. Both of these reactions proceed with good to excellent diastereo- and enantioselectivity using a single class of gold catalysts, namely C(2)-symmetric bis(phosphinegold(I) carboxylate) complexes. The development of the azlactone Mannich reaction to provide fully protected anti-α,β-diamino acid derivatives is described. 1,3-Dipolar cycloaddition reactions of several acyclic 1,2-disubstituted alkenes and the chemistry of the resultant cycloadducts are examined to probe the stereochemical course of this reaction. Reaction kinetics and tandem mass spectrometry studies of both the cycloaddition and Mannich reactions are reported. These studies support a mechanism in which the gold complexes catalyze addition reactions through nucleophile activation rather than the more typical activation of the electrophilic reaction component.
中文翻译:

金 (I) 催化二氢唑酮的非对映和对映选择性 1,3-偶极环加成和曼尼希反应
吖内酯参与与缺电子烯烃和N-磺酰醛亚胺的立体选择性反应,分别产生1,3-偶极环加成和曼尼希加成反应的产物。这两个反应均使用一类金催化剂,即 C(2)-对称双(膦金(I)羧酸酯)络合物,以良好至优异的非对映选择性和对映选择性进行。描述了二氢唑酮曼尼希反应的发展,以提供完全保护的抗 α,β-二氨基酸衍生物。研究了几种无环 1,2-二取代烯烃的 1,3-偶极环加成反应以及所得环加合物的化学性质,以探讨该反应的立体化学过程。报告了环加成反应和曼尼希反应的反应动力学和串联质谱研究。这些研究支持了一种机制,其中金络合物通过亲核试剂活化而不是更典型的亲电子反应组分的活化来催化加成反应。
更新日期:2011-03-16
中文翻译:

金 (I) 催化二氢唑酮的非对映和对映选择性 1,3-偶极环加成和曼尼希反应
吖内酯参与与缺电子烯烃和N-磺酰醛亚胺的立体选择性反应,分别产生1,3-偶极环加成和曼尼希加成反应的产物。这两个反应均使用一类金催化剂,即 C(2)-对称双(膦金(I)羧酸酯)络合物,以良好至优异的非对映选择性和对映选择性进行。描述了二氢唑酮曼尼希反应的发展,以提供完全保护的抗 α,β-二氨基酸衍生物。研究了几种无环 1,2-二取代烯烃的 1,3-偶极环加成反应以及所得环加合物的化学性质,以探讨该反应的立体化学过程。报告了环加成反应和曼尼希反应的反应动力学和串联质谱研究。这些研究支持了一种机制,其中金络合物通过亲核试剂活化而不是更典型的亲电子反应组分的活化来催化加成反应。































 京公网安备 11010802027423号
京公网安备 11010802027423号