当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Unnatural Carbamate-Protected α-Alkyl Amino Esters via N–H Bond Insertion Reactions
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-10-13 , DOI: 10.1021/acscatal.2c03937
You Li 1 , Yu-Xuan Su 1 , Yu-Tao Zhao 1 , Lu Liu 1 , Mao-Lin Li 1 , Shou-Fei Zhu 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-10-13 , DOI: 10.1021/acscatal.2c03937
You Li 1 , Yu-Xuan Su 1 , Yu-Tao Zhao 1 , Lu Liu 1 , Mao-Lin Li 1 , Shou-Fei Zhu 1, 2
Affiliation
|
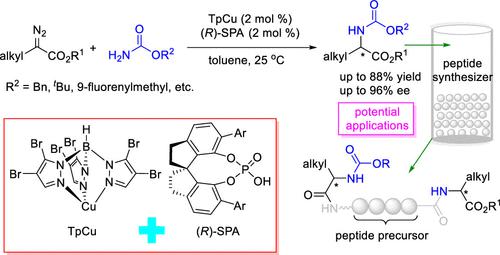
We have developed a method for highly enantioselective N–H bond insertion reactions of α-alkyl-α-diazoacetates and various carbamates cooperatively catalyzed by TpCu, where Tp = (hydrotris(4,5-bromopyrazolyl)borate), and a chiral spiro phosphoric acid. These reactions provide straightforward access to unnatural optically active N-carbonate α-alkyl-α-amino esters, which are widely used in artificial peptide synthesis. This mild method uses readily available starting materials and features a broad substrate scope, good functional group tolerance, good to high yields (58–88%), and high enantioselectivities (90–96% ee). The combination of the neutral copper catalyst TpCu and the chiral spiro phosphoric acid ensures the success of the reaction by inhibiting a β-H migration reaction of a copper carbene intermediate and by promoting enantioselective proton transfer to an enolate intermediate.
中文翻译:

通过 N-H 键插入反应选择性合成非天然氨基甲酸酯保护的 α-烷基氨基酯
我们开发了一种由 TpCu 协同催化的 α-烷基-α-重氮乙酸酯和各种氨基甲酸酯的高度对映选择性 N-H 键插入反应的方法,其中 Tp = (hydrotris(4,5-bromopyrazolyl)borate) 和手性螺环磷酸酯酸。这些反应提供了直接获得非自然光学活性N-碳酸酯α-烷基-α-氨基酯,广泛用于人工肽合成。这种温和的方法使用容易获得的起始材料,具有广泛的底物范围、良好的官能团耐受性、良好的高产率 (58-88%) 和高对映选择性 (90-96% ee)。中性铜催化剂 TpCu 和手性螺磷酸的组合通过抑制铜卡宾中间体的 β-H 迁移反应和促进对映选择性质子转移到烯醇化物中间体来确保反应的成功。
更新日期:2022-10-13
中文翻译:

通过 N-H 键插入反应选择性合成非天然氨基甲酸酯保护的 α-烷基氨基酯
我们开发了一种由 TpCu 协同催化的 α-烷基-α-重氮乙酸酯和各种氨基甲酸酯的高度对映选择性 N-H 键插入反应的方法,其中 Tp = (hydrotris(4,5-bromopyrazolyl)borate) 和手性螺环磷酸酯酸。这些反应提供了直接获得非自然光学活性N-碳酸酯α-烷基-α-氨基酯,广泛用于人工肽合成。这种温和的方法使用容易获得的起始材料,具有广泛的底物范围、良好的官能团耐受性、良好的高产率 (58-88%) 和高对映选择性 (90-96% ee)。中性铜催化剂 TpCu 和手性螺磷酸的组合通过抑制铜卡宾中间体的 β-H 迁移反应和促进对映选择性质子转移到烯醇化物中间体来确保反应的成功。

































 京公网安备 11010802027423号
京公网安备 11010802027423号