当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A convenient total synthesis of indolo[2,3-b]carbazole-6,12-dione
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2022-10-11 , DOI: 10.1002/jhet.4585 Suchandra Chakraborty 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2022-10-11 , DOI: 10.1002/jhet.4585 Suchandra Chakraborty 1
Affiliation
|
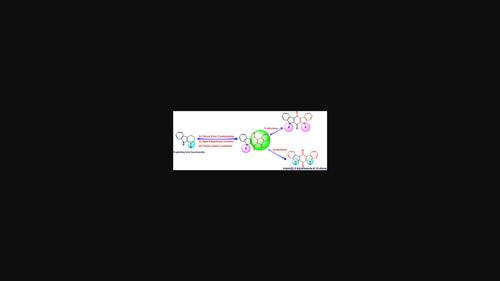
Indolo[2,3-b]carbazole-6,12-dione is exceptionally attractive due to its efficient antitumor activity. Synthesis of biologically potent symmetric indolo[2,3-b]carbazole-6,12-dione and its N-alkylated derivatives has been achieved by exploring the keto group of easily accessible 2,3,4,9-tetrahydro-1H-carbazol-1-one with remarkable yields just in five steps, utilizing the two classical reactions—Japp-Klingemann procedure followed by Fischer Indole Cyclisation as key steps. The required formylation of N-alkylated 2,3,4,9-tetrahydro-1H-carbazol-1-one has been effectively accomplished through Cross Claisen Condensation.
中文翻译:

吲哚[2,3-b]咔唑-6,12-二酮的简便全合成
Indolo[2,3-b]carbazole-6,12-dione 由于其有效的抗肿瘤活性而极具吸引力。通过探索易于获得的 2,3,4,9-tetrahydro-1 H的酮基,已经实现了具有生物学效用的对称吲哚并[2,3-b]咔唑-6,12-二酮及其N-烷基化衍生物的合成- carbazol-1-one 只需五个步骤即可获得显着的产率,利用两个经典反应——Japp-Klingemann 程序,然后是 Fischer Indole Cyclisation 作为关键步骤。N-烷基化 2,3,4,9-四氢-1 H-咔唑-1-酮所需的甲酰化已通过交叉克莱森缩合有效地完成。
更新日期:2022-10-11
中文翻译:

吲哚[2,3-b]咔唑-6,12-二酮的简便全合成
Indolo[2,3-b]carbazole-6,12-dione 由于其有效的抗肿瘤活性而极具吸引力。通过探索易于获得的 2,3,4,9-tetrahydro-1 H的酮基,已经实现了具有生物学效用的对称吲哚并[2,3-b]咔唑-6,12-二酮及其N-烷基化衍生物的合成- carbazol-1-one 只需五个步骤即可获得显着的产率,利用两个经典反应——Japp-Klingemann 程序,然后是 Fischer Indole Cyclisation 作为关键步骤。N-烷基化 2,3,4,9-四氢-1 H-咔唑-1-酮所需的甲酰化已通过交叉克莱森缩合有效地完成。












































 京公网安备 11010802027423号
京公网安备 11010802027423号