当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tacticity and Ionization Effects on Adsorption Behavior of Poly(acrylic acid) and Poly(methacrylic acid) at the CCl4–H2O Interface Revealed by MD Simulations
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-10-12 , DOI: 10.1021/acs.iecr.2c02416 Raviteja Kurapati 1 , Upendra Natarajan 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-10-12 , DOI: 10.1021/acs.iecr.2c02416 Raviteja Kurapati 1 , Upendra Natarajan 1
Affiliation
|
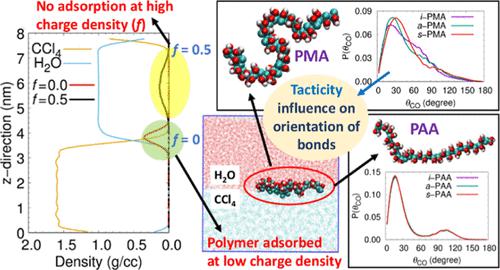
Atomistic molecular dynamic simulations were performed to investigate the adsorption behavior of poly(acrylic acid) (PAA) and poly(methacrylic acid) (PMA) at the CCl4–H2O interface for isotactic, atactic, and syndiotactic forms. The conformational, orientation, and solvation behaviors of PAA and PMA chains at the interface were studied as a function of the degree of ionization (f). The calculated density profiles show that adsorption occurs only when degree of ionization is less than a critical value (ionization of 20% groups). The density profiles of different groups show the existence of carboxylic acid and carboxylate groups toward the aqueous phase and methyl groups toward the oil phase, relative to the interface. The radius of gyration values and dihedral distributions of completely adsorbed chains (i.e., for f = 0) reveal their existence in an extended conformation at the interface, in contrast to their coiled structure in bulk aqueous solution. The size of adsorbed chains (f < 0.2) decreases with increase in degree-of-ionization due to looping of chain toward water; the extent of looping depends on the distribution of charge on the chain. The carbonyl and methyl groups of uncharged PAA and PMA show two set of orientations corresponding to direction toward water and oil phases and this preferential orientation decreases with increase in degree-of-ionization. Significant differences in orientation distribution, dihedral angle, and hydration were observed among different tacticities of PMA which primarily reflect the hydrophobic nature of isotactic PMA as compared to other tacticity. The number of hydrogen bonds between the polyelectrolyte and water is much lower at the interface relative to the bulk aqueous phase as determined by the population of water in the interface region as well as charge on the polyelectrolyte.
中文翻译:

MD 模拟揭示了聚(丙烯酸)和聚(甲基丙烯酸)在 CCl4-H2O 界面吸附行为的策略性和电离效应
进行原子分子动力学模拟以研究聚(丙烯酸)(PAA)和聚(甲基丙烯酸)(PMA)在 CCl 4 -H 2 O 界面对等规、无规和间规形式的吸附行为。研究了界面处 PAA 和 PMA 链的构象、取向和溶剂化行为与电离度 ( f)。计算的密度分布表明吸附仅在电离度小于临界值(20% 基团的电离)时发生。不同基团的密度分布表明,相对于界面,存在朝向水相的羧酸和羧酸盐基团以及朝向油相的甲基。完全吸附链的回转值半径和二面角分布(即,对于f = 0)揭示了它们在界面处以扩展构象的存在,这与它们在本体水溶液中的盘绕结构形成对比。吸附链的大小 ( f< 0.2) 由于链环向水,随着电离度的增加而降低;循环的程度取决于链上电荷的分布。不带电荷的 PAA 和 PMA 的羰基和甲基基团显示出两组取向,对应于朝向水相和油相的方向,并且这种优先取向随着电离度的增加而降低。在不同立构规整度的 PMA 中观察到取向分布、二面角和水合度的显着差异,这主要反映了等规立构的疏水性PMA 与其他立构规整度相比。聚电解质和水之间的氢键数量在界面处相对于本体水相要低得多,这由界面区域中的水数量以及聚电解质上的电荷确定。
更新日期:2022-10-12
中文翻译:

MD 模拟揭示了聚(丙烯酸)和聚(甲基丙烯酸)在 CCl4-H2O 界面吸附行为的策略性和电离效应
进行原子分子动力学模拟以研究聚(丙烯酸)(PAA)和聚(甲基丙烯酸)(PMA)在 CCl 4 -H 2 O 界面对等规、无规和间规形式的吸附行为。研究了界面处 PAA 和 PMA 链的构象、取向和溶剂化行为与电离度 ( f)。计算的密度分布表明吸附仅在电离度小于临界值(20% 基团的电离)时发生。不同基团的密度分布表明,相对于界面,存在朝向水相的羧酸和羧酸盐基团以及朝向油相的甲基。完全吸附链的回转值半径和二面角分布(即,对于f = 0)揭示了它们在界面处以扩展构象的存在,这与它们在本体水溶液中的盘绕结构形成对比。吸附链的大小 ( f< 0.2) 由于链环向水,随着电离度的增加而降低;循环的程度取决于链上电荷的分布。不带电荷的 PAA 和 PMA 的羰基和甲基基团显示出两组取向,对应于朝向水相和油相的方向,并且这种优先取向随着电离度的增加而降低。在不同立构规整度的 PMA 中观察到取向分布、二面角和水合度的显着差异,这主要反映了等规立构的疏水性PMA 与其他立构规整度相比。聚电解质和水之间的氢键数量在界面处相对于本体水相要低得多,这由界面区域中的水数量以及聚电解质上的电荷确定。































 京公网安备 11010802027423号
京公网安备 11010802027423号