当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
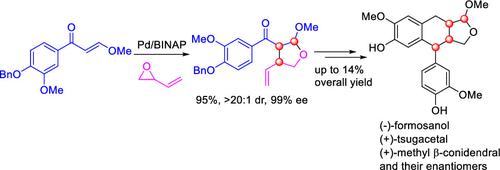
Stereoselective Total Synthesis of Formosanol, Tsugacetal, and Methyl β-Conidendral
Organic Letters ( IF 4.9 ) Pub Date : 2022-10-12 , DOI: 10.1021/acs.orglett.2c03159 Meiqi Li 1 , Yiming Liu 1 , Huiyu Si 2 , Xin Zhou 2 , Yong Jian Zhang 1, 3
Organic Letters ( IF 4.9 ) Pub Date : 2022-10-12 , DOI: 10.1021/acs.orglett.2c03159 Meiqi Li 1 , Yiming Liu 1 , Huiyu Si 2 , Xin Zhou 2 , Yong Jian Zhang 1, 3
Affiliation
|
The first enantioselective total synthesis of aryltetralin lignan acetals, (−)-formosanol, (+)-tsugacetal, (+)-methyl β-conidendral, and their enantiomers have been accomplished on the basis of the Pd-catalyzed asymmetric allylic cycloaddition as a key step. Six stereoisomers of the lignan acetals have been synthesized via a 7–8 step sequence in up to 14% overall yield. The in vitro cytotoxicity against several cancer cells has preliminarily been examined for the obtained six stereoisomers of lignan acetals.
中文翻译:

甲醛醇、Tsugacetal 和甲基 β-Conidendral 的立体选择性全合成
以Pd催化的不对称烯丙基环加成反应为基础,首次对映选择性全合成了芳基四氢萘木脂素缩醛、(−)-甲醛醇、(+)-杉缩醛、(+)-甲基β-异构体及其对映体。关键一步。通过 7-8 个步骤序列合成了木脂素缩醛的六种立体异构体,总产率高达 14%。初步研究了所得木脂素缩醛的六种立体异构体对多种癌细胞的体外细胞毒性。
更新日期:2022-10-12
中文翻译:

甲醛醇、Tsugacetal 和甲基 β-Conidendral 的立体选择性全合成
以Pd催化的不对称烯丙基环加成反应为基础,首次对映选择性全合成了芳基四氢萘木脂素缩醛、(−)-甲醛醇、(+)-杉缩醛、(+)-甲基β-异构体及其对映体。关键一步。通过 7-8 个步骤序列合成了木脂素缩醛的六种立体异构体,总产率高达 14%。初步研究了所得木脂素缩醛的六种立体异构体对多种癌细胞的体外细胞毒性。












































 京公网安备 11010802027423号
京公网安备 11010802027423号